High-risk endometrial cancer may be benefit from adjuvant radiotherapy plus chemotherapy
Objective: To present patterns of practice and outcomes in the adjuvant treatment of intermediate- and
high-risk endometrial cancer.
Methods: Retrospective data on 224 women with intermediate-risk and high-risk endometrial cancer
from 1999 to 2006 were reviewed. All patients underwent surgical staging. Patterns of adjuvant treatment,
consisting of pelvic radiotherapy, chemotherapy, and radiotherapy plus chemotherapy, were assessed. The 3-
and 5-year disease-specific survival (DSS) rates were calculated using the Kaplan-Meier method.
Results: The difference in 5-year DSS rate was statistically significant between adjuvant group and
non-adjuvant group (80.65% vs. 63.80%, P=0.040). In 110 high-risk patients who underwent adjuvant
treatment, both 5-year DSS rate and recurrent rate were significantly different in combined radiotherapy
and chemotherapy group compared with radiotherapy alone and chemotherapy alone groups (DSS rate,
P=0.049; recurrent rate, P=0.047). In 83 intermediate-risk women who underwent adjuvant treatment, there
was no significant difference in 5-year DSS rate and recurrence rate among the combined radiotherapy
and chemotherapy, radiotherapy alone and chemotherapy alone groups (DSS rate, P=0.776; recurrent rate,
P=0.937).
Conclusions: Adjuvant radiotherapy plus chemotherapy is associated with a higher 5-year DSS rate and
lower recurrence rate compared with radiotherapy alone and chemotherapy alone in high-risk endometrial
cancer patients. Patients with intermediate-risk endometrial cancer may be not likely to benefit from
adjuvant combined radiotherapy and chemotherapy.
Key words: Adjuvant treatment; chemotherapy; endometrial cancer; radiotherapy; recurrence
Introduction
Endometrial cancer is one of the most common gynecologic malignancies, with an ever-increasing incidence (1). Although consensus on surgical staging has been reached, postoperative adjuvant treatment remains controversial. Major prognostic factors are stage, histological type, grade, depth of myometrial invasion, and lymph-vascular space invasion (LVSI). Adjuvant treatment has been tailored to these risk factors.
Radiation therapy (RT) is the conventional postoperative adjuvant treatment for patients with intermediate- and high-risk endometrial cancer. However, a number of studies have indicated that postoperative adjuvant pelvic external beam radiotherapy (EBRT) or vaginal brachytherapy (VBT) reduced the local recurrence rate without increasing the overall survival (OS) rate (2,3). In the past 20 years, much attention has been paid to postoperative chemotherapy (CHEMO), concurrent RT and CHEMO, as well as combined RT and CHEMO (RT&CHEMO). However, relatively little is known regarding regimen selection and efficacy of chemotherapy-containing adjuvant treatments.
The aim of this study was to explore the feasibility, effectivity and toxicity of adjuvant treatment for intermediate- and high-risk endometrial cancer.
Materials and methods
General information
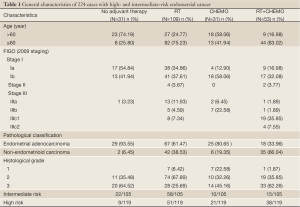
This study included 224 patients with intermediate-risk and high-risk factors treated from January 1999 to December 2006 in Beijing Obstetrics and Gynecology Hospital, of which, 193 cases received postsurgical treatment (adjuvant treatment group) and 31 patients did not undergo any adjuvant treatment (non-adjuvant treatment group). Surgical staging [International Federation of Gynaecology and Obstetrics (FIGO) 2009], including extrafascial total abdominal hysterectomy, bilateral salpingo-oophorectomy, and pelvic lymphadenectomy (PLN)/para-aortic lymph node dissection, was administered in all patients. The intermediate-risk group (105 cases) included patients with stage IA G3 or other non-endometrial adenocarcinoma pathological type; stage IB, G1 and G2, with endometrioid type histology (4). The high-risk group (119 cases) are stage IB of grade 3 or of non-endometrioid histology; or stage II or III (4).
Adjuvant treatment: postoperative pelvic EBRT with or without VBT (RT group)
A total of 162 patients (including 109 RT alone and 53 RT+CHEMO cases) were treated with EBRT, of which 148 (91.36%) had pelvic standard rectangular field RT and 14 (8.64%) received BOX field RT, with a dose range of 40-45 Gy. Four Patients (2.47%) with positive common iliac lymph nodes or para-aortic lymph nodes were assigned to concurrent pelvic irradiation and para-abdominal aortic extended field irradiation with a dose range of 40-45 Gy, and 7 patients (4.32%) with parametrial metastasis or local pelvic lymph node metastasis were given the above pelvic EBRT and subsequent reduced field irradiation covering the parametrial region or lymph node metastatic region with the dose range of 5-10 Gy. Ten patients (6.17%) including 6 intermediate-risk patients and 4 high-risk patients consented to receive EBRT followed by immediate VBT with a dose range of 10-20 Gy. None of the patients in this study underwent postoperative VBT alone.
Postoperative CHEMO
Among 84 patients (45.52%, including 31 CHEMO alone and 53 RT+CHEMO cases) who were assigned to postoperative systemic CHEMO, 31 (36.90%) adopted 4-6 cycles of CHEMO, 32 (38.10%) underwent 2-3 cycles of CHEMO followed by RT (CHEMO-RT); 3 (3.57%) received RT followed by 2-3 cycles of CHEMO (RT-CHEMO); 9 (10.71%) had 2-3 cycles of CHEMO first and then RT followed by same regimen CHEMO for 2-3 cycles (CHEMO-RT-CHEMO); 3 (3.57%) received RT plus 2-3 cycles of CHEMO at the same time (RT+CHEMO); and 6 (7.14%) underwent 2-3 cycles of CHEMO first followed by RT plus the same CHEMO regimen at the same time for 2-3 cycles (CHEMO-CHEMO+RT). CHEMO was delivered at 21-d intervals. The regimens were: cisplatin, doxorubicin plus cyclophosphamide (PAC) for 36 patients (42.86%); cisplatin plus paclitaxel (PT) for 33 patients (39.29%); cisplatin plus doxorubicin (PA) for 10 patients (11.90%); cisplatin, vincristine plus doxorubicin (PVA) for 1 patient (1.19%); cisplatin, ifosfamide plus doxorubicin (PIA) for 1 patient (1.19%); cisplatin, etoposide plus cyclophosphamide (PEC) for 1 patient (1.19%); cisplatin plus cyclophosphamide (PC) for 1 patient (1.19%); and cyclophosphamide, doxorubicin plus 5-fluorouracil (CAF) for 1 patient (1.19%).
Follow up
The median follow-up time was 49 months (range, 3-130 months). Fifty-nine (26.34%) of the 224 patients were lost during the follow-up period. Survival time was calculated as the time from diagnosis to death or the last follow up. Disease-specific survival (DSS) time was calculated under the following situations: patients were died of diseases, or patients’ death was caused by treatment-induced complications directly or indirectly.
Statistical analysis
SP.SS 17.0 statistical software package (SPSS Inc., Chicago, IL, USA) was adopted for the statistical analysis. The Kaplan-Meier method was used to estimate the survival rates and log-rank test was used to analyze the significance. Comparison of rates including recurrence rates and toxicity rates was performed by Pearson Chi-square and continuity correction test.
Results
General characteristics
The study included 224 patients with a median age of 57 years (range, 23-75 years). Of the 224 patients, 109 (48.66%) received RT alone postoperatively, 31 (13.84%) received postoperative CHEMO alone, and 53 (23.66%) received postoperative RT plus CHEMO. Stage and CHEMO-RT sequences are showed in Tables 1,2.
Full table
Full table
DSS and recurrence rates
The 3- and 5-year DSS rates were 90.09% and 80.65%, respectively, in adjuvant treatment group, and 80.2% and 63.80%, respectively, in non-adjuvant treatment group. The difference in 5-year DSS was statistically significant between the two groups (Kaplan-Meier log-rank, P=0.040).
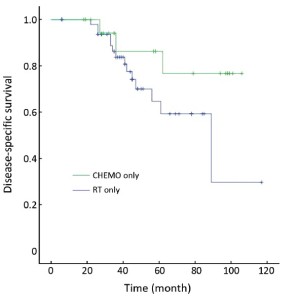
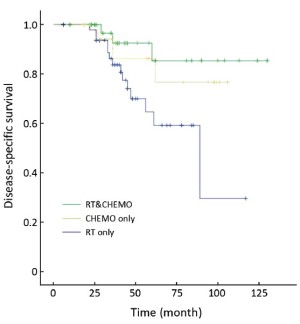
Table 3 showed that the DSS rate was significantly different among the three different adjuvant treatment groups (Kaplan-Meier log-rank, P=0.049) in high-risk patients, in which the difference between RT&CHEMO and RT alone groups was statistically significant (Kaplan-Meier log-rank, P=0.017), whereas, the other two comparisons were not significant (RT&CHEMO vs. CHEMO: Kaplan-Meier log-rank, P=0.503; RT vs. CHEMO alone: Kaplan-Meier log-rank, P=0.135). The survival curves are shown in Figures 1-4. The 5-year DSS rates were not significantly different among patients who received different sequences of RT plus CHEMO (Kaplan-Meier log-rank, P=0.455).
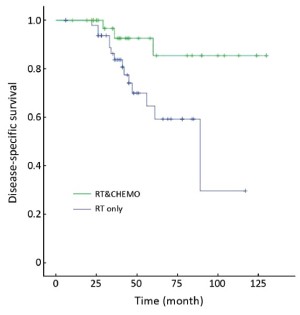
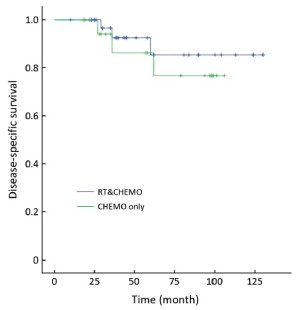
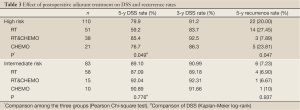
Full table
In intermediate-risk group, the DSS rate was not significantly different among RT alone, CHEMO alone and RT&CHEMO groups (Kaplan-Meier log-rank, P=0.776). The 5-year recurrence rate was not significantly different among these three treatment groups (Pearson Chi-square test, P=0.937).
During treatment, 5 (2.59%) of 193 patients had progressive disease, in which 3 underwent simple RT, 1 received CHEMO alone and the remaining 1 patient received combined RT and CHEMO (CHEMO-RT).
Toxicity of adjuvant treatment
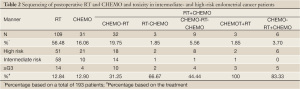
Forty-two patients (21.76%) experienced ≥ grade 3 (G3+) toxicity (Table 2). G3+ toxicity of RT+CHEMO/CHEMO-RT+CHEMO and sequential CHEMO-RT-CHEMO patients was significantly higher than that in CHEMO-RT/RT-CHEMO and RT alone patients (66.67% vs. 18.06%, continuity correction test, P=0.001). G3+ toxicity of CHEMO-RT/RT-CHEMO was significantly higher than that of RT alone (34.28% vs. 12.84%, Continuity Correction test, P=0.004).
Twenty-four patients stopped treatment in the middle of RT due to G3+ toxicity. Such experience happened in 8 patients (7.34%) undergoing RT alone, 4 patients (12.50%) undergoing CHEMO-RT, 2 patients (66.67%) undergoing RT-CHEMO, 2 patients (22.22%) undergoing CHEMO-RT-CHEMO, 3 patients (100%) undergoing RT+CHEMO, and 5 patients (83.33%) undergoing CHEMO-RT+CHEMO. The percentage of patients who stopped RT halfway was significantly higher in groups of combined RT and CHEMO than RT alone (30.19% vs. 7.34%, continuity correction test, P=0.001), whereas there was no significant difference between CHEMO-RT group and simple RT group. (7.34% vs. 12.50%, continuity correction, P=0.576).
Discussion
Despite many years of exploration into the postoperative treatment for intermediate- and high-risk endometrial cancer, more work is needed.
Guidelines from the National Comprehensive Cancer Network (NCCN) and other studies have suggested that postoperative pelvic EBRT or VBT is helpful in reducing local recurrence (pelvic and vaginal cuff) in most patients with intermediate- and high-risk endometrial cancer (5-10). The GOG-99 trial has shown that adjuvant pelvic RT can reduce the relapse rate in intermediate-risk patients, but that the OS time was not extended (11). Other studies have reported 5-year survival rates in the range of 84-89% in patients with intermediate-risk endometrial cancer who received postoperative RT (4,8,12-14). In our study, in intermediate-risk patients who received RT alone, the 3- and 5-year DSS rates were 90.99% and 89.10%, respectively, similar to the results of the above mentioned studies.
Side effects of postoperative pelvic EBRT are marked, particularly in the small intestine (4,7,15). In our study, the G3+ side effect rate in patients receiving RT alone was 12.84%; in fact, 7.34% of the patients had to stop RT halfway.
GOG-122 and other studies suggested that high-risk patients would benefit more from postoperative CHEMO than from pelvic RT (16-22). However, researchers suggested that neither progression-free survival nor OS was significantly different in patients with high-risk endometrial cancer treated with postoperative adjuvant CHEMO compared with RT (23). In our study, 3- and 5-year DSS rates went up to 86.3% and 76.7% in patients assigned to postsurgical CHEMO alone, compared with 83.7% and 59.2%, respectively, in patients treated with postoperative RT alone, even these differences were not statistically significant. The recurrence rate in CHEMO group was not significantly lower compared with RT alone.
Postoperative RT does not improve OS and postoperative CHEMO alone has higher local recurrence rate in patients with high-risk endometrial cancer. Therefore, much attention has been paid to combined postoperative RT and CHEMO (18,24-32). The most recent research showed that the addition of CHEMO to RT improved the rate of clinical benefit from 55% to 77% (20). However, no large multicenter study has reported on the efficacy of combined RT and CHEMO, selection of the most appropriate CHEMO regimen, and most appropriate sequence combination for RT and CHEMO (CHEMO-RT, RT-CHEMO, RT+CHEMO, or the “sandwich” program of CHEMO-RT-CHEMO). Our retrospective analysis indicated that patients with high-risk cancer treated with combined RT and CHEMO had better DSS rates and lower recurrence rate. In this combined RT and CHEMO group, the sequence of CHEMO-RT has fewer side effects than other combining sequence, whereas, the benefit was not presented in intermediate-risk patients.
We propose that the treatment for high-risk endometrial cancer patients with combined adjuvant RT and CHEMO can lead to a reduction in recurrence rate as well as improvements in the DSS rate, compared with postoperative RT alone and CHEMO alone. The beneficial trend for postoperative RT combined with CHEMO found in this non-randomized study should be further examined in a large randomized study.
Acknowledgements
This work was supported by Funds of Capital Medical Development Foundation (2007-1049).
Disclosure: The authors declare no conflict of interest.
References
- Bertz J, Dahm S, Haberland J, et al. Verbreitung von Krebserkrankungen in Deutschland -Entwicklung der Prävalenzen zwischen 1990 und 2010. In: Robert Koch-Institut (Hrsg), Beiträge zur Gesundheitsberichterstattung des Bundes. Berlin: Robert Koch-Institut. 2012.
- Scholten AN, van Putten WL, Beerman H, et al. Postoperative radiotherapy for Stage 1 endometrial carcinoma: long-term outcome of the randomized PORTEC trial with central pathology review. Int J Radiat Oncol Biol Phys 2005;63:834-8.
- ASTEC/EN.5 Study Group, Blake P, Swart AM, et al. Adjuvant external beam radiotherapy in the treatment of endometrial cancer (MRC ASTEC and NCIC CTG EN.5 randomised trials): pooled trial results, systematic review, and meta-analysis. Lancet 2009;373:137-46.
- Creutzberg CL, Nout RA. The role of radiotherapy in endometrial cancer: current evidence and trends. Curr Oncol Rep 2011;13:472-8.
- National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology. Available online: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. (Accessed May 12, 2009).
- Nout RA, Smit VT, Putter H, et al. Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high-intermediate risk (PORTEC-2): an open-label, non-inferiority, randomised trial. Lancet 2010;375:816-23.
- Nout RA, Putter H, Jürgenliemk-Schulz IM, et al. Quality of life after pelvic radiotherapy or vaginal brachytherapy for endometrial cancer: first results of the randomized PORTEC-2 trial. J Clin Oncol 2009;27:3547-56.
- Kong A, Johnson N, Kitchener HC, et al. Adjuvant radiotherapy for stage I endometrial cancer. Cochrane Database Syst Rev 2012;3:CD003916.
- Abu-Rustum NR, Chi DS, Leitao M, et al. What is the incidence of isolated paraaortic nodal recurrence in grade 1 endometrial carcinoma? Gynecol Oncol 2008;111:46-8.
- Zuliani AC, Cairo AA, Esteves SC, et al. Adjuvant radiotherapy in early stage endometrial cancer. Rev Assoc Med Bras 2011;57:438-42.
- Keys HM, Roberts JA, Brunetto VL, et al. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol Oncol 2004;92:744-51.
- Lin LL, Mutch DG, Rader JS, et al. External radiotherapy versus vaginal brachytherapy for patients with intermediate risk endometrial cancer. Gynecol Oncol 2007;106:215-20.
- Röper B, Astner ST, Heydemann-Obradovic A, et al. Ten-year data on 138 patients with endometrial carcinoma and postoperative vaginal brachytherapy alone: no need for external-beam radiotherapy in low and intermediate risk patients. Gynecol Oncol 2007;107:541-8.
- Lee NK, Cheung MK, Shin JY, et al. Prognostic factors for uterine cancer in reproductive-aged women. Obstet Gynecol 2007;109:655-62.
- Nout RA, Putter H, Jürgenliemk-Schulz IM, et al. Five-year quality of life of endometrial cancer patients treated in the randomised Post Operative Radiation Therapy in Endometrial Cancer (PORTEC-2) trial and comparison with norm data. Eur J Cancer 2012;48:1638-48.
- Miller DS. Combination chemotherapy in treating patients with stage III, stage IV, or recurrent endometrial cancer 2009. ClinicalTrials.gov. Available online: http://clinicaltrials.gov/ct2/show/NCT00063999.
- Klopp AH, Jhingran A, Ramondetta L, et al. Node-positive adenocarcinoma of the endometrium: outcome and patterns of recurrence with and without external beam irradiation. Gynecol Oncol 2009;115:6-11.
- Hogberg T, Rosenberg P, Kristensen G, et al. A randomized phase III study on adjuvant treatment with radiation (RT) ± chemotherapy (CT) in early stage high-risk endometrial cancer (NSGO-EC-9501/EORTC 55991). J Clin Oncol 2007;25:5503.
- Randall ME, Filiaci VL, Muss H, et al. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol 2006;24:36-44.
- Schwandt A, Chen WC, Martra F, et al. Chemotherapy plus radiation in advanced-stage endometrial cancer. Int J Gynecol Cancer 2011;21:1622-7.
- Susumu N, Sagae S, Udagawa Y, et al. Randomized phase III trial of pelvic radiotherapy versus cisplatin-based combined chemotherapy in patients with intermediate- and high-risk endometrial cancer: a Japanese Gynecologic Oncology Group study. Gynecol Oncol 2008;108:226-33.
- Tewari KS, Filiaci VL, Spirtos NM, et al. Association of number of positive nodes and cervical stroma invasion with outcome of advanced endometrial cancer treated with chemotherapy or whole abdominal irradiation: a Gynecologic Oncology Group study. Gynecol Oncol 2012;125:87-93.
- Maggi R, Lissoni A, Spina F, et al. Adjuvant chemotherapy vs radiotherapy in high-risk endometrial carcinoma: results of a randomised trial. Br J Cancer 2006;95:266-71.
- Homesley HD, Filiaci V, Gibbons SK, et al. A randomized phase III trial in advanced endometrial carcinoma of surgery and volume directed radiation followed by cisplatin and doxorubicin with or without paclitaxel: A Gynecologic Oncology Group study. Gynecol Oncol 2009;112:543-52.
- Yan X, Gao M, Jiang GQ, et al. Adjuvant chemotherapy as postoperative treatment for high-risk stage I endometrial cancer. Bei Jing Yi Xue (in Chinese) 2011;33:1-4.
- Ding YH, Ma YB. Clinical analysis of postoperative chemotherapy on patients with high-risk early stage endometrial cancer. Zhonghua Zhong Liu Fang Zhi Za Zhi 2009; 16:1892-3.
- Ren YL, Wang HY, Shi DR, et al. Combined treatment and prognostic factors for stage III and IV endometrial carcinoma. Zhonghua Fu Chan Ke Za Zhi 2008;43:523-7.
- Scribner DR Jr, Puls LE, Gold MA. A phase II evaluation of docetaxel and carboplatin followed by tumor volume directed pelvic plus or minus paraaortic irradiation for stage III endometrial cancer. Gynecol Oncol 2012;125:388-93.
- Abaid LN, Rettenmaier MA, Brown JV 3rd, et al. Sequential chemotherapy and radiotherapy as sandwich therapy for the treatment of high risk endometrial cancer. J Gynecol Oncol 2012;23:22-7.
- Marchetti C, Pisano C, Mangili G, et al. Use of adjuvant therapy in patients with FIGO stage III endometrial carcinoma: a multicenter retrospective study. Oncology 2011;81:104-12.
- Lupe K, D’Souza DP, Kwon JS, et al. Adjuvant carboplatin and paclitaxel chemotherapy interposed with involved field radiation for advanced endometrial cancer. Gynecol Oncol 2009;114:94-8.
- McMeekin DS. Pelvic radiation therapy or vaginal implant radiation therapy, paclitaxel, and carboplatin in treating patients with high-risk stage I or stage II endometrial cancer. 2011. ClinicalTrials.gov. Available online: http://clinicaltrials.gov/ct2/show/NCT00807768