Melatonin inhibits the expression of vascular endothelial growth factor in pancreatic cancer cells
Objective: To investigate the effects of melatonin on cellular proliferation and endogenous vascular
endothelial growth factor (VEGF) expression in pancreatic carcinoma cells (PANC-1).
Methods: PANC-1 cells were cultured for this study. The secreted VEGF concentration in the culture
medium was determined using ELISA method, VEGF production in the tumor cells was detected by
immunocytochemistry, and VEGF mRNA expression was determined by RT-PCR.
Results: Higher melatonin concentrations significantly inhibited cellular proliferation, with 1 mmol/
L concentration exhibiting the highest inhibitory effect (P<0.01). VEGF concentrations in the cell culture
supernatants and intra-cellules were all significantly reduced after melatonin (1 mmol/L) incubation (P<0.05).
VEGF mRNA expression decreased markedly in a time-dependent manner during the observation period (P<0.05).
Conclusions: High melatonin concentrations markedly inhibited the proliferation of pancreatic carcinoma
cells. The endogenous VEGF expression was also suppressed by melatonin incubation.
Key words: Melatonin; VEGF; pancreatic cancer
Introduction
Pancreatic cancer is now the fifth leading cause of cancer in the United States, Japan, and Europe, with an overall five-year survival rate of <5% (1). One of the major reasons for this serious situation is the peritoneal dissemination and early metastasis of pancreatic cancer, such that most patients present with an already unresectable malignant tumor upon diagnosis (2). Akin to other solid tumors, an adequate blood supply is the key factor in the tumor development of pancreatic cancer, through angiogenesis at both the primary and secondary cancer sites. Thus, anti-angiogenesis therapy has been a challenging target for tumor therapeutics because it theoretically offers long-term control of tumor progression (3).
Vascular endothelial growth factor (VEGF), the most active endogenous pro-angiogenic factor and an endothelial cell-specific mitogen, is involved in angiogenesis in various types of tumors, including pancreatic cancer (4). Several studies have discovered a very close correlation between VEGF and pancreatic cancer development (5-8). In pancreatic cancer cells, both VEGF and VEGF receptors (VEGFR) are overexpressed. VEGF promotes cancer growth, dissemination, and metastasis, and its expression level is positively correlated with the prognosis of pancreatic cancer in diagnosed patients or animal models. In pre-clinical and clinical studies, anti-VEGF therapy has a direct role against VEGF in tumor development; it also potently inhibits tumor cell growth, which results in significant improvement of treatment response, patient survival, and progression-free survival. Therefore, VEGF-targeted therapeutic agents are a pivotal research focus, and are a prospective strategy in the treatment of diseases, especially cancer.
Melatonin, the major secretory product of the pineal gland, is considered the most important natural oncostatic hormone in the human body, which reduces the incidence and growth of tumors based on compelling evidence (9-11). Nevertheless, the oncostatic mechanism of melatonin is still obscure. The immunomodulatory, anti-proliferative, and anti-oxidant effects of melatonin explain its oncostatic activity. Even the possible anti-angiogenic effect of melatonin may also be involved in this activity. Some significant basic clinical studies (12,13) have shown that melatonin-induced control of neoplastic growth is associated with a decline in VEGF secretion, which suggests that melatonin could exert its anti-angiogenic activity indirectly through its inhibition of VEGF expression. However, the abovementioned studies are very preliminary. The aim of the present study is to determine the melatonin effects on pancreatic cancer cells and to explore in detail the oncostatic mechanism of melatonin.
Materials and methods
Cell culture
Pancreatic carcinoma cells (PANC-1), a human pancreatic cancer cell line, were obtained from the Cell Biology Center of the Chinese Academy of Medical Sciences and the Peking Union Medical College. The cells were cultured in Dulbecco’s modified eagle medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum, 4 mmol/L glutamine, 100 nmol/L sodium pyruvate, 25 mmol/L HEPES, 100 U/mL penicillin, and 100 U/mL streptomycin, and incubated at 37 ℃ in a humidified atmosphere containing 5% CO2. Cells in good condition were selected for the succeeding study.
Cell proliferation and cell viability assay
Cell proliferation was determined by 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method. The cells were seeded at a density of 5,000-10,000 cells/well on a 96-well culture plate. According to the experimental design, after 24 h, the culture medium respectively containing 1, 10, 100, 1,000, 10,000, 100,000 and 1,000,000 nmol/L melatonin (Sigma, St. Louis, USA) was added to the different pancreatic cell groups for a time range from 0 h to 24 h. Thereafter, the incubation environment was kept away from light. Approximately 24 h later, 20 µL of 5 mg/mL MTT was added to each well of the 96-well plate. The cells were continuously incubated for 4 h, and then the medium was removed and replaced with 150 µL dimethyl sulfoxide (DMSO) per well. The absorbance was measured at 490 nm wavelength using an enzyme-linked immunosorbent assay (ELISA) plate reader (BioRad, Benchmark, Japan). The samples were assayed with eight replicates, and the mean optical density (OD) values were analyzed. Cell viability was determined by trypan blue exclusion test. The same experiments were performed at least three times.
Determination of VEGF concentration in cultured supernatants by ELISA
PANC-1 cells were seeded on 60 mm diameter dishes and continually incubated for 24 h. Then, 1 mmol/L melatonin was added to the experiment group. After 48 h of incubation away from light, the VEGF concentrations secreted by PANC-1 cells in the supernatants of the experimental group and the untreated control were quantified by ELISA. The cultured supernatant was collected and centrifuged at 12,000 r/min at 4 ℃ for 15 min, and then ELISA was performed according to the manufacturer’s instructions (VEGF ELISA kit, Promega, Madison, USA). The OD values (A450) were measured at a wavelength of 450 nm. The total cell number was counted using the cell-counting plate for at least three times. The standard curve was determined by SPSS statistical software (SPSS Inc., Chicago, IL, USA). The supernatants were harvested with six replicates, and the experiment was performed three times.
Detection of VEGF expression in cultured PANC-1 cells in vitro by immunocytochemistry
Cells at 105 cells/well were seeded on a 24-well culture plate to detect VEGF expression in the PANC-1 cells. Approximately 24 h later, the culture medium was replaced with conditioned medium with or without 1 mmol/L melatonin. The cells were incubated further at 37 ℃ in a CO2 incubator under a light-free environment for 24 h. After fixation with buffered paraformaldehyde, the cells were treated with pronase for 30 min, incubated with 0.3% H2O2 in methanol for 30 min to eliminate the endogenous peroxide activity, rinsed with phosphate buffered saline (PBS), and then incubated with normal blocking serum for 20 min. Afterward, the cells were incubated overnight with VEGF-A mouse-anti-human polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, USA) at 4 ℃. Thereafter, the bridging antibody [poly-horse radish peroxide (HRP) anti-mouse IgG; Santa Cruz Biotechnology, Santa Cruz, USA] was incubated with the cells for 20 min at 30 ℃. Finally, 3,3'-diaminobenzidine (DAB) was applied according to the manufacturer’s protocol (DAB Kit, Promega, Madison, USA). For the image analysis, 150 cells, each with a clear outline, from 10 microscopic fields (15 cells in each field) were selected randomly from each group under 200× magnification. The average gray value and integral OD of each group were automatically measured using HPIAS-1000, a high resolution pathological image analysis system.
Detection of VEGF mRNA expression by reverse transcription polymerase chain reaction (RT-PCR)
PANC-1 cells were seeded on 60 mm diameter dishes, incubated for 24 h, and starved for another 24 h. Then, the medium with 1 mmol/L melatonin was added. After 24 h incubation, total RNA was extracted separately from the cells of each group with TRIzol® reagent following the manufacturer’s instructions. Approximately 2 µg total RNA sample [treated in 8 mL diethylpyrocarbonate (DEPC) water in an Eppendorf centrifuge (Ep) tube and to which 1 µL of 10 mmol/L dNTPmix and 1 µL of 0.5 µg/µL Oligo(dt)12-18 were added] was denaturalized by incubating at 65 ℃ for 5 min. The Ep tube was placed in an ice bath for 2 min. The RNA sample was then reverse-transcribed to complementary DNA (cDNA) as follows: the denatured RNA was incubated at 42 ℃ for 2 min with 2 µL of 10× RT buffer, 4 µL of 25 mmol/L MgCl2, 2 µL of 0.1 mol/L dithiothreitol (DTT), and 1 µL of 50 U/µL recombinant RNase inhibitor (RNaseOUTTM). Then, 1 µL (50 units) SuperScriptTM II reverse transcriptase was added, followed by incubation at 42 ℃ for 50 min and termination at 70 ℃ for 15 min in a total volume of 20 µL. Finally, after chilled on ice, 1 µL RNase H was added, and then the solution was incubated for 20 min at 37 ℃ before PCR was performed. For the PCR, 2 µL of the resulting cDNA, 36.75 µL of tripled-distilled water, 5 µL of 10× PCR buffer, 3 µL of 25 mmol/L MgCl2, 1 µL of 10 mmol/L dNTPs, 1 µL each of the 10 µmol/L sense and antisense primers, and 0.25 µL of 5 U/µL Taq DNA polymerase in a total volume of 50 µL were added. The samples were amplified through incubation at 94 ℃ for 5 min, 30 cycles of denaturation at 94 ℃ for 45 s, annealing at 54 ℃ for 45 s and extension at 72 ℃ for 1 min, and final incubation at 72 ℃ for 7 min. The PCR products were analyzed on 1% agarose gel containing ethidium bromide. The primer sequences for VEGF165 were sense: 5'-GGGCAGAATCATCACGAAGT-3' and antisense: 5'-AAATGCTTTCTCCGCTCTGA-3' (359 bp); and for b-actin, sense: 5'-GTGCGTGACATTAAGGAG-3' and antisense: 5'-CTAAGTCATAGTCCGCCT-3' (520 bp).
Statistical analysis
Statistical analysis was performed using the SPSS version 10.0 statistical package for Windows (SPSS Inc. Chicago, IL, USA). All data were presented as x±s. The t-test was used for the comparison between the groups of cell proliferation, mRNA, and VEGF expression. Correlation regression was used for the correlation between the OD values and VEGF concentrations in the cultured medium. P<0.05 was considered statistically significant.
Results
MTT and cell viability test
After 24 h of incubation, lower melatonin concentrations (1 nmol/L-1,000 nmol/L) had no obvious effect on cellular proliferation (P>0.05). However, higher melatonin concentrations (≥100,000 nmol/L) significantly inhibited cell proliferation, especially when the melatonin concentration reached 1 mmol/L (P<0.01) (Figure 1A). At different time points, 1 mmol/L melatonin treatment on PANC-1 cells induced cell growth down-regulation, which was detectable after 3 h of incubation (P<0.05) and increased with treatment time (at 24 h, P<0.01) (Figure 1B). Using the trypan blue exclusion test, the cell viability assay demonstrated that after melatonin treatment, the PANC-1 cells in each group had >98% viability, indicating that 1 mmol/L melatonin had no evident toxic effects, in agreement with our previous study. Moreover, no quantitative evidence of acute cytotoxicity was detected, as determined by the cell viability assay. Therefore, in this experiment, 1 mmol/L melatonin showed the greatest anti-proliferative effect on PANC-1 cells, exhibiting enhanced performance with increased treatment time.
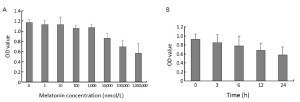
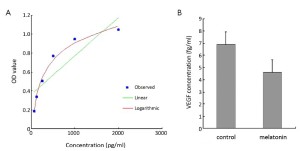
Effect of melatonin on VEGF production in cultured supernatants
After 24 h incubation, the VEGF concentration in the cultured supernatant was significantly decreased in the experimental group (0.006,91±0.001,31 pg/cell, P<0.05) compared with the control group (0.004,61±0.000,926 pg/cell, P<0.05), which indicated that melatonin could inhibit VEGF production in PANC-1 cells (Figure 2A,B).
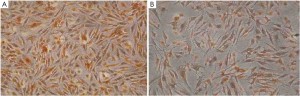
Immunocytochemical staining of VEGF protein expression in PANC-1 cells
From the immunocytochemical staining, VEGF positive staining is clearly seen in the cell cytoplasm and membrane, and shows a markedly deceased VEGF expression compared with the control group (the average gray value: 45.72±7.85 vs. 91.28±14.56, P<0.05) (Figure 3A,B).
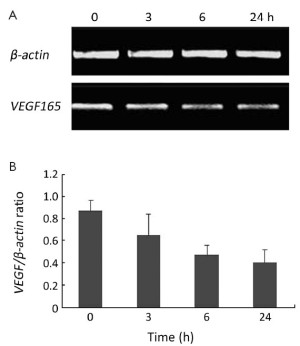
Effect of melatonin on VEGF mRNA expression in PANC-1 cells
According to the human VEGF165 cDNA record in GenBank and the trait of the designed specific primers, the whole length of the RT-PCR products identified by agarose gel electrophoresis was accorded with the background reference of the human VEGF165 cDNA (Figure 4A,B). Furthermore, the PCR products were determined by DNA sequence analysis and contained the full length of VEGF165 mRNA similar to that described in GenBank with accession number AF-486837. Based on the RT-PCR analysis results, VEGF mRNA expression was significantly decreased in the melatonin group after 6 h and 24 h treatment (P<0.05), suggesting that melatonin could suppress VEGF mRNA transcription.
Discussion
Angiogenesis, which is the sprouting of new blood vessels from the existing endothelium, is essential for wound repair, organ regeneration, embryonic vascular system development, and a variety of pathological conditions, especially tumor angiogenesis. Tumor growth, development, and behavior are dependent on angiogenesis, especially solid tumors (14,15). Increased angiogenesis is associated with tumor metastases, poor prognosis, and reduced patient survival (15-17). The tumor cell properties of releasing and inducing several angiogenic and antiangiogenic factors play a crucial role in regulating endothelial cell (EC) proliferation, migration, apoptosis or survival, and cell-cell and cell-matrix adhesion through different intracellular signals, and are the essential mechanisms of tumor induced-angiogenesis (17,18). Theoretically, if antiangiogenic agents are administered before a tumor develops or becomes dependent on a vascular supply, they would act similar to a vaccine in preventing tumor development and tumor growth (15,17,19). Therefore, understanding the angiogenesis-regulating mechanism could provide new therapeutic options for cancer treatment.
Considering that VEGF, the most important mediator of tumor angiogenesis, is crucial to pancreatic cancer development and extensive vascularization (17), in our experiment, VEGF was selected as a reliable parameter to ascertain whether melatonin has an anti-angiogenic effect on pancreatic cancer cells in vitro. In our experiment, high levels of VEGF protein and mRNA expression were detected in the control group. This finding agreed with previous studies and supported the view that pancreatic carcinoma has extensive tumor neoangiogenesis and extensive vascularization, which is correlated with the overexpression of several angiogenic factors, such as VEGF, platelet-derived endothelial cell growth factor, and matrix metalloproteinases (18-20). In immunochemistry detection, the VEGF was also markedly detected in the tumor cell cytoplasm, suggesting that VEGF is not just a secreted mediator active in the extracellular environment through the VEGF-VEGFR system.
Being the most important naturally oncostatic hormone, melatonin possibly exerts its anti-tumor effect through the immunomodulatory, anti-proliferative or anti-oxidant pathway (9,21-24). However, in this experiment, melatonin exhibited a possible anti-angiogenetic mechanism. Previous studies have demonstrated the anti-angiogenic action of melatonin on tumor angiogenesis, either directly or indirectly (13,25,26). In the present study, after 1 mmol/L melatonin administration, a significant decrease of VEGF concentration in the culture supernatants was observed compared with the control group, demonstrating an evident inhibitory effect of melatonin on VEGF mRNA expression. In addition, from the immunocytochemistry study, the VEGF intracellular localization also decreased in the melatonin group. Based on these results, 1 mmol/L melatonin could significantly inhibit VEGF production in PANC-1 cells, thus suggesting its possible anti-angiogenic effect.
How do melatonin and VEGF interact? This issue was not tackled in detail in this experiment. The immuno-enhancing activity of melatonin could possibly explain the mechanism for melatonin and VEGF interaction, considering that VEGF suppresses the immune response by blocking dendritic cell maturation (27). From a physiopathological point of view, melatonin may be involved in the regulation of neoangiogenesis due to its modulatory role in immunity and hematopoiesis (28,29). Moreover, melatonin receptors, retinoid Z receptor/retinoid orphan receptor, and other mechanisms are involved in the neoangiogenesis process (30,31), which will be an important focus of our future research. In our previous study, we found that high concentrations of melatonin inhibited elevated cell proliferation and cell migration of the human umbilical vein endothelial cells stimulated by co-culturing with PANC-1 cells through the suppression of VEGF expression in PANC-1 cells (32). Through the anti-proliferative effect (as confirmed by many studies and by this present experiment), but not the possible anti-angiogenic effect (which was not proven in detail elsewhere except in this study) of melatonin, the melatonin action is dependent on the cell type, functional cellular state or several other factors, such as the drug concentration in this experiment (10,33). Melatonin has a marked antitumor effect in the concentration range from nmol/L to several hundreds mmol/L (34,35). In our study, melatonin exhibited a great inhibitory effect on VEGF production when in high concentrations (1 mmol/L). Medical therapeutics using low melatonin concentrations may become realistic goals in the future.
In summary, our study showed that high concentrations of melatonin inhibited the cellular proliferation of pancreatic carcinoma cells and suppressed endogenous VEGF expression, therefore melatonin may be used in very innovative and challenging antiangiogenesis therapeutics of cancers.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 30901745).
Disclosure: The authors declare no conflict of interest.
References
- Schönau KK, Steger GG, Mader RM. Angiogenic effect of naive and 5-fluorouracil resistant colon carcinoma on endothelial cells in vitro. Cancer Lett 2007;257:73-8.
- Niedergethmann M, Alves F, Neff JK, et al. Gene expression profiling of liver metastases and tumour invasion in pancreatic cancer using an orthotopic SCID mouse model. Br J Cancer 2007;97:1432-40.
- Xie K, Wei D, Huang S. Transcriptional anti-angiogenesis therapy of human pancreatic cancer. Cytokine Growth Factor Rev 2006;17:147-56.
- Thurston G, Kitajewski J. VEGF and Delta-Notch: interacting signalling pathways in tumour angiogenesis. Br J Cancer 2008;99:1204-9.
- Doi Y, Yashiro M, Yamada N, et al. Significance of phospho-vascular endothelial growth factor receptor-2 expression in pancreatic cancer. Cancer Sci 2010;101:1529-35.
- Liang QL, Wang BR, Chen GQ, et al. Clinical significance of vascular endothelial growth factor and connexin43 for predicting pancreatic cancer clinicopathologic parameters. Med Oncol 2010;27:1164-70.
- Li K, Li MJ, He T, et al. Expression of vascular endothelial growth factor C in pancreatic cancer and its effect upon lymph node metastasis. Zhonghua Yi Xue Za Zhi 2009;89:2386-90.
- Ochi N, Matsuo Y, Sawai H, et al. Vascular endothelial growth factor-C secreted by pancreatic cancer cell line promotes lymphatic endothelial cell migration in an in vitro model of tumor lymphangiogenesis. Pancreas 2007;34:444-51.
- Reiter RJ. Mechanisms of cancer inhibition by melatonin. J Pineal Res 2004;37:213-4.
- Sainz RM, Mayo JC, Rodriguez C, et al. Melatonin and cell death: differential actions on apoptosis in normal and cancer cells. Cell Mol Life Sci 2003;60:1407-26.
- Cabrera J, Negrín G, Estévez F, et al. Melatonin decreases cell proliferation and induces melanogenesis in human melanoma SK-MEL-1 cells. J Pineal Res 2010;49:45-54.
- Lissoni P, Rovelli F, Malugani F, et al. Anti-angiogenic activity of melatonin in advanced cancer patients. Neuro Endocrinol Lett 2001;22:45-7.
- Dai M, Cui P, Yu M, et al. Melatonin modulates the expression of VEGF and HIF-1 alpha induced by CoCl2 in cultured cancer cells. J Pineal Res 2008;44:121-6.
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1995;1:27-31.
- Cook KM, Figg WD. Angiogenesis inhibitors: current strategies and future prospects. CA Cancer J Clin 2010;60:222-43.
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature 2000;407:249-57.
- Carmeliet P. VEGF as a key mediator of angiogenesis in cancer. Oncology 2005;69 Suppl 3:4-10.
- Gupta MK, Qin RY. Mechanism and its regulation of tumor-induced angiogenesis. World J Gastroenterol 2003;9:1144-55.
- Saif MW. Anti-angiogenesis therapy in pancreatic carcinoma. JOP J Pancreas (Online) 2006;7:163-73.
- Turner HE, Harris AL, Melmed S, et al. Angiogenesis in endocrine tumors. Endocr Rev 2003;24:600-32.
- Reiter RJ, Tan DX, Erren TC, et al. Light-mediated perturbations of circadian timing and cancer risk: a mechanistic analysis. Integr Cancer Ther 2009;8:354-60.
- Carrillo-Vico A, Guerrero JM, Lardone PJ, et al. A review of the multiple actions of melatonin on the immune system. Endocrine 2005;27:189-200.
- Carbajo-Pescador S, García-Palomo A, Martín-Renedo J, et al. Melatonin modulation of intracellular signaling pathways in hepatocarcinoma HepG2 cell line: role of the MT1 receptor. J Pineal Res 2011;51:463-71.
- Vijayalaxmi, Thomas CR Jr, Reiter RJ, et al. Melatonin: from basic research to cancer treatment clinics. J Clin Oncol 2002;20:2575-601.
- Cui P, Luo Z, Zhang H, et al. Effect and mechanism of melatonin’s action on the proliferation of human umbilical vein endothelial cells. J Pineal Res 2006;41:358-62.
- Cui P, Yu M, Luo Z, et al. Intracellular signaling pathways involved in cell growth inhibition of human umbilical vein endothelial cells by melatonin. J Pineal Res 2008;44:107-14.
- Di Nicola M, Anichini A, Mortarini R, et al. Human dendritic cells: natural adjuvants in antitumor immunotherapy. Cytokines Cell Mol Ther 1998;4:265-73.
- Silva SO, Ximenes VF, Livramento JA, et al. High concentrations of the melatonin metabolite, N1-acetyl-N2-formyl-5-methoxykynuramine, in cerebrospinal fluid of patients with meningitis: a possible immunomodulatory mechanism. J Pineal Res 2005;39:302-6.
- Sutherland ER, Martin RJ, Ellison MC, et al. Immunomodulatory effects of melatonin in asthma. Am J Respir Crit Care Med 2002;166:1055-61.
- Cho SY, Lee HJ, Jeong SJ, et al. Sphingosine kinase 1 pathway is involved in melatonin-induced HIF-1α inactivation in hypoxic PC-3 prostate cancer cells. J Pineal Res 2011;51:87-93.
- Zhang D, Li B, Shi J, et al. Suppression of tumor growth and metastasis by simultaneously blocking vascular endothelial growth factor (VEGF)-A and VEGF-C with a receptor-immunoglobulin fusion protein. Cancer Res 2010;70:2495-503.
- Cui P, Yu M, Peng X, et al. Melatonin prevents human pancreatic carcinoma cell PANC-1-induced human umbilical vein endothelial cell proliferation and migration by inhibiting vascular endothelial growth factor expression. J Pineal Res 2012;52:236-43.
- Pawlikowski M, Winczyk K, Karasek M. Oncostatic action of melatonin: facts and question marks. Neuro Endocrinol Lett 2002;23:24-9.
- García-Santos G, Antolín I, Herrera F, et al. Melatonin induces apoptosis in human neuroblastoma cancer cells. J Pineal Res 2006;41:130-5.
- Weinreb O, Mandel S, Youdim MB. cDNA gene expression profile homology of antioxidants and their antiapoptotic and proapoptotic activities in human neuroblastoma cells. FASEB J 2003;17:935-7.


