NQO1 C609T polymorphism correlated to colon cancer risk in farmers from western region of Inner Mongolia
Objective: To investigate the relationship between NAD(P)H:quinone oxidoreductase 1 (NQO1) C609T
polymorphism and colon cancer risk in farmers from western region of Inner Mongolia.
Methods: Polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) was
performed to analyze NQO1 C609T polymorphism from 160 healthy controls and 76 colon cancer patients.
Results: Among the colon cancer patients, the incidence of NQO1 T allele (53.29%) was significantly higher
than it in control group (33.75%, P<0.001). The individuals with NQO1 T allele had higher risk [2.239 (95% CI:
1.510-3.321) times] to develop colon cancer than individuals with NQO1 C allele. The incidence of NQO1
(T/T) (34.21%) in colon cancer patients was higher than that in control group (15.62%, PNQO1 (T/T) and NQO1 (T/C) genotype carriers had 3.813 (95% CI: 1.836-7.920) times
and 2.080 (1.026-4.219) times risk compared with wild-type NQO1 (C/C) gene carriers in developing colon cancer.
Individuals with NQO1 (T/T) genotype had 2.541 (95% CI: 0.990-6.552) times, 3.713 (95% CI: 1.542-8.935) times,
and 3.471 (95% CI: 1.356-8.886) times risk than individuals with NQO1 (T/C) or NQO1 (C/C) genotype in welldifferentiated,
moderately-differentiated, and poorly-differentiated colon cancer patients, respectively.
Conclusions: NQO1 gene C609T could be one of risk factors of colon cancer in farmers from western
region of Inner Mongolia.
Key words: Colon cancer; NAD(P)H:quinone oxidoreductase 1; gene polymorphism; Inner Mongolia
Introduction
Quinone is a noxious chemical compound, existing generally through nature, in exhaust of automobile, benzene, tobacco smoke, which is involved in development of tumor, mutation and necrosis in mammalian cells. NAD(P)H: quinone oxidoreductase 1 (NQO1) catalyzes two-electron reduction of quinones and nitrogen oxides (1) and turns quinones to hydroquinones directly. Most hydroquinones are more stable and can be metabolized within cells. Therefore, NQO1 can prevent organs from carcinogen-induced tumorigenesis. Previous studies (2,3) showed that NQO1 has protected against carcinogenesis and mutation induced by quinone and its derivants. Studies (4-6) also found that the expression of NQO1 is elevated in many tumor cells and human cancer tissues, suggesting that NQO1 might be the target of drugs for cancer therapy. Polymorphism of NQO1 gene occurs at the site of 609 with cytosine changed into thymine. Although it would not affect synthesis of RNA, the protein product of NQO1 T609 is ubiquitinated and subsequently degraded, which may lead to null NQO1 (7). Our previous study (8) showed that the incidence of NQO1 C609T in gastric cancer patients is higher than that in controls. Individuals with NQO1 (T/C) and NQO1 (T/T) genotypes have higher onset risk with odds ratios (OR) of 2.080 and 3.813 respectively.
Colon cancer is one of the most common cancers, and it is the third cause of cancer related death. Various factors, multiple genes and pathways are involved in colon carcinogenesis and progress. K-ras, adenomatous polyposis coli (APC), tumor protein P53 (TP53) and mismatch repair (MMR) genes (9-12), chromosomal instability, microsatellite instability (13,14) and wnt-hedgehog pathway (15,16) are reported to be involved in initiation and progress of colon cancer. Diet factors and carcinogens play roles in influencing onset risk of colon cancer (17,18). The molecular mechanism of colon cancer has been better understood, however, the prognosis of colon cancer has not been improved too much. Therefore we carried out a case-control study in farmers from western region of Inner Mongolia to examine the association between NQO1 C609T polymorphism and risk of developing colon cancer. Farmers from local region have their unique eating habits. They consume lots of pickled meat and vegetables which contain high levels of salt and nitrite. They are used to have the animal oils. The diet mostly consists of grain and less fresh fruits and vegetables. Most of them are alcohol users. As a result of specific diet of farmers, we study the NQO1 polymorphism and susceptibility to colon cancer, which may also improve the treatment of local colon cancer patients.
Materials and methods
Subjects
All 76 colon cancer patients were hospitalized cases from the Affiliated Hospital of Inner Mongolia Medical College from 2007-2008, which were all diagnosed by histopathological examination, and did not undergo anti-cancer treatment. Of the 76 patients, 46 were male and 30 were female, with a mean age of 60.13±10.96 years (range, 39-79 years). One hundred and sixty healthy individuals who matched the age and sex of the colon cancer patients were selected by routine physical examination. The healthy controls did not have either tumors, tumor-related diseases detected by physical examination, or history of familial colon cancer. All individuals involved in the study gave informed consent.
DNA extraction and polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP)
DNA was extracted from 1 ml venous blood of the investigated objects, and then PCR was performed with the primers of 5'-AAGCCCAGACCAACTTCA-3' and 5'-GCGTTTCTTCCATCCTT-3' and under following conditions: initial denaturation at 94 ℃ for 5 min, followed by 33 circles of denaturation at 94 ℃ for 1min, annealing at 54 ℃ for 45 s and extension at 72 ℃ for 45 s, with a final extension of 10 min at 72 ℃. The PCR products were digested with restriction enzyme Hinf I. The full size of PCR product was 195 bp. There was no enzyme site of HinfI in PCR product of wild-type NQO1 (C/C). If NQO1 C609→T base substitution occurred, the PCR product was digested into two fragments (119 bp and 76 bp). There were 2 fragments in NQO1 (T/T) and 3 fragments in NQO1 (T/C).
Statistical analysis
The frequency of NQO1 (C/C), NQO1 (T/C) and NQO1 (T/T) was compared in control group vs. colon cancer patients and according to clinical characteristics by Chi-square test. OR [95% confidence interval (95% CI)] was calculated. All the statistical analyses were performed by SPSS 11.1 statistic software (SPSS Inc., Chicago, IL, USA).
Results
We investigated the incidence of NQO1 T609 allele in colon cancer patients and control group. We found that the incidence of NQO1 T609 allele in colon cancer patients was 53% which was significantly higher than it in control group (33.75%). The individuals with NQO1 T609 allele had higher risk [2.239 (95% CI: 1.510-3.321) times] to develop colon cancer compared to individuals with NQO1 C609 allele.
Distribution of NQO1 C609T polymorphism was different between colon cancer patients and control group. In the control group, the frequency of NQO1 (C/C), NQO1 (T/C) and NQO1 (T/T) was 48.13%, 36.25% and 15.62%, respectively. While in colon cancer patients, the frequency of NQO1 (C/C), NQO1 (T/C) and NQO1 (T/T) was 27.63%, 38.16%, and 34.21% (Table 1), respectively. There was a significantly increased incidence of NQO1 (T/T) in colon cancer patients compared with control group, (χ2=13.566, P<0.001), while the incidence of NQO1 (T/C) was not increased in colon cancer patients compared with control group. OR analysis showed that individuals with NQO1 (T/T) or NQO1 (T/C) genotype had higher risk [3.813 (95% CI: 1.836-7.920) time and 2.080 (95% CI: 1.026-4.219) time respectively] to develop colon cancer than individuals with wild type NQO1 (C/C) (Table 1).
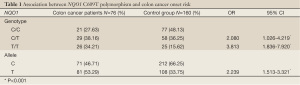
Full table
We investigated the distribution of NQO1 C609T polymorphism in the differentiation of colon cancer. The frequency of NQO1 (T/T) was found that it is increased in colon cancer patients with the increased malignance (32.00% in well-differentiated colon cancer patients, 40.00% in moderately-differentiated colon cancer patients and 42.00% in poorly-differentiated colon cancer patients). In well-differentiated colon cancer patients, NQO1 (T/T) gene carriers had 2.541 times (95% CI: 0.990-6.522 times) higher chance in developing colon cancer than NQO1 (T/C) and NQO1 (C/C) gene carriers, which was more than the risk in control group (χ2=3.956, P<0.05). As for moderately-differentiated colon cancer patients, individuals with NQO1 (T/T) genotype had a higher onset risk than individuals with NQO1 (T/C) or NQO1 (C/C) genotype with OR of 3.713 (95% CI: 1.542-8.935), which was more risk than the control group (χ2=9.374, P<0.01). In poorly-differentiated colon cancer patients, NQO1 (T/T) gene carriers had much higher onset risk than NQO1 (C/C) and NQO1 (T/C) gene carriers with OR of 3.471 (95% CI: 1.356-8.886), which was significantly more risk than the control group (χ2=7.345, P<0.01) (Table 2). With the decreasing differentiation of colon cancer, the patients with NQO1 (T/T) genotype were increased, indicating that NQO1 C609T polymorphism may be involved in the progress of colon cancer.

Full table
Discussion
Studies about the association between NQO1 C609T polymorphism and cancers have been carried out. The results are controversial. Some studies showed that NQO1 C609T polymorphism is correlated with several types of cancers, including esophageal (19,20), gastric (19,21), renal (22), breast (23), cervical (24), bladder(25) and urothelial (22-26) cancers. In contrast, there is no correlation between NQO1 C609T polymorphism and esophageal (27), gastric (27), renal (28), breast (27,29), hepatocellular (30), head and neck (31) cancers. NQO1 *2/*2 is associated with poor survival in non-small cell lung cancer patients (32). These results indicated that the inactive NQO1 may affect the risk of developing cancers depending on the type of cancer, ethnic group, diet factors and carcinogen exposure.
Previous studies (2,3) showed that NQO1 could inhibit colon carcinogenesis at both initiation and post initiation stages in carcinogens induced Sprague-Dawley rat colon cancer model. There was an increase frequency of NQO1*2/*2 genotype in colon cancer patients compared with control group. However, the frequency of NQO1*1/*2 did not show increasing in colon cancer patients compared with control group. As far as tobacco and alcohol use concerned, the incidence of NQO1*2/*2 genotype was almost same in tobacco and alcohol users and nonusers, indicating that the consumption of tobacco and alcohol was not associated with NQO1 C609T polymorphism. Using colon cancer patients and age, gender matched healthy individuals from local region, our study found that NQO1 C609T polymorphism was correlated with colon cancer onset risk. Our result was consistent with other studies (33-35).
To further elucidate the association between NQO1 C609T polymorphism and colon cancer, we investigated the correlation between NQO1 C609T polymorphism and the differentiation of colon cancer. We found that the frequency of NQO1 (T/T) was increased in colon cancer patients with the increased malignance, and the patients with NQO1 (T/T) had higher onset risk to develop colon cancer in well-differentiated, moderately-differentiated and poorly-differentiated colon cancer, which suggested that NQO1 may play an role in prevention colon carcinogenesis and progress. The increasing trend of NQO1 (T/T) frequency did not show any significant difference between differentiation of colon cancer, which may due to the small numbers of colon cancer patients involved. Therefore, larger numbers of patients will be enrolled to demonstrate our result in the future study.
Hypoxia of tumor cells is one of the most significant reasons in failure of radiotherapy and chemotherapy. Drugs such as reductants have a specific cytotoxicity to hypoxia cells. This kind of chemicals forms the anionic clusters which can get electrons after reduction in cells. These anionic clusters have high reaction with oxygen. Under hypoxia, these anionic clusters have toxicity, or develop into metabolic products with toxicity. Lots of anticancer drugs containing quinone are used in clinical treatment such as “mitomycin” (36). Our study finds that the control group has 15.62% NQO1 (T/T) genotype, while colon cancer patients have 34.21% NQO1 (T/T) genotype. Our studies indicated that over one third of patients have no NQO1 activity. It is necessary to identify the right population of cancer patients to be treated with the drug containing quinone. Alternatively we also can treat this part of patients with other kinds of anticancer drugs. It is reported that “lochnerol” and dimethylfumarate (DFM) can induce the activity of enzymes like NQO1 (37,38), so we also can treat cancer patients with NQO1 (T/C) genotype with “lochnerol” and special dietary combined with other anticancer drugs to improve the effect of chemotherapy. Based on our data, identifying the polymorphism of NQO1 for cancer patients has an important role in choosing anticancer drugs for clinical treatment.
In conclusion, after studying 160 healthy controls and 76 colon cancer patients, we found that there was a significantly increased incidence of NQO1 T609 allele in patients with colon carcinoma compared with it in control group. NQO1 T609 allele carriers had higher risk than NQO1 C 609 allele carriers to develop colon carcinoma.
Acknowledgements
Our research is supported by the Autonomous region Natural Science Foundation of China, No. 200711020906.
Disclosure: The authors declare no conflict of interest.
References
- Riley RJ, Workman P. DT-diaphorase and cancer chemotherapy. Biochem Pharmacol 1992;43:1657-69.
- Begleiter A, Sivananthan K, Curphey TJ, et al. Induction of NAD(P)H quinone: oxidoreductase1 inhibits carcinogen-induced aberrant crypt foci in colons of Sprague-Dawley rats. Cancer Epidemiol Biomarkers Prev 2003;12:566-72.
- Begleiter A, Sivananthan K, Lefas GM, et al. Inhibition of colon carcinogenesis by post-initiation induction of NQO1 in Sprague-Dawley rats. Oncol Rep 2009;21:1559-65.
- Marín A, López de Cerain A, Hamilton E, et al. DT-diaphorase and cytochrome B5 reductase in human lung and breast tumours. Br J Cancer 1997;76:923-9.
- Siegel D, Ross D. Immunodetection of NAD(P)H:quinone oxidoreductase 1 (NQO1) in human tissues. Free Radic Biol Med 2000;29:246-53.
- Choudry GA, Stewart PA, Double JA, et al. A novel strategy for NQO1 (NAD(P)H:quinone oxidoreductase, EC 1.6.99.2) mediated therapy of bladder cancer based on the pharmacological properties of EO9. Br J Cancer 2001;85:1137-46.
- Siegel D, Anwar A, Winski SL, et al. Rapid polyubiquitination and proteasomal degradation of a mutant form of NAD(P)H:quinone oxidoreductase 1. Mol Pharmacol 2001;59:263-8.
- Ren JJ, OuYang XH, Su XL. NAD(P)H:quinone oxidoreductase gene polymorphism association with gastric carcinoma. Zhong Hua Zhong liu Fang Zhi Za Zhi (in Chinese) 2006;22:1686-8.
- Gervaz P, Bouzourene H, Cerottini JP, et al. Dukes B colorectal cancer: distinct genetic categories and clinical outcome based on proximal or distal tumor location. Dis Colon Rectum 2001;44:364-72; discussion 372-3.
- Kikuchi H, Pino MS, Zeng M, et al. Oncogenic KRAS and BRAF differentially regulate hypoxia-inducible factor-1alpha and -2alpha in colon cancer. Cancer Res 2009;69:8499-506.
- Lee SH, Lee SJ, Chung JY, et al. p53, secreted by K-Ras-Snail pathway, is endocytosed by K-Ras-mutated cells; implication of target-specific drug delivery and early diagnostic marker. Oncogene 2009;28:2005-14.
- Phelps RA, Chidester S, Dehghanizadeh S, et al. A two-step model for colon adenoma initiation and progression caused by APC loss. Cell 2009;137:623-34.
- Patchett SE, Alstead EM, Saunders BP, et al. Regional proliferative patterns in the colon of patients at risk for hereditary nonpolyposis colorectal cancer. Dis Colon Rectum 1997;40:168-71.
- Miyakura Y, Sugano K, Akasu T, et al. Extensive but hemiallelic methylation of the hMLH1 promoter region in early-onset sporadic colon cancers with microsatellite instability. Clin Gastroenterol Hepatol 2004;2:147-56.
- Frattini M, Balestra D, Suardi S, et al. Different genetic features associated with colon and rectal carcinogenesis. Clin Cancer Res 2004;10:4015-21.
- Bian YH, Huang SH, Yang L, et al. Sonic hedgehog-Gli1 pathway in colorectal adenocarcinomas. World J Gastroenterol 2007;13:1659-65.
- Doll R, Peto R. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J Natl Cancer Inst 1981;66:1191-308.
- Kim YI, Mason JB. Nutrition chemoprevention of gastrointestinal cancers: a critical review. Nutr Rev 1996;54:259-79.
- Sarbia M, Bitzer M, Siegel D, et al. Association between NAD(P)H: quinone oxidoreductase 1 (NQ01) inactivating C609T polymorphism and adenocarcinoma of the upper gastrointestinal tract. Int J Cancer 2003;107:381-6.
- Zhang J, Schulz WA, Li Y, et al. Association of NAD(P)H: quinone oxidoreductase 1 (NQO1) C609T polymorphism with esophageal squamous cell carcinoma in a German Caucasian and a northern Chinese population. Carcinogenesis 2003;24:905-9.
- Zhang JH, Li Y, Wang R, et al. NQO1 C609T polymorphism associated with esophageal cancer and gastric cardiac carcinoma in North China. World J Gastroenterol 2003;9:1390-3.
- Schulz WA, Krummeck A, Rösinger I, et al. Increased frequency of a null-allele for NAD(P)H: quinone oxidoreductase in patients with urological malignancies. Pharmacogenetics 1997;7:235-9.
- Menzel HJ, Sarmanova J, Soucek P, et al. Association of NQO1 polymorphism with spontaneous breast cancer in two independent populations. Br J Cancer 2004;90:1989-94.
- Niwa Y, Hirose K, Nakanishi T, et al. Association of the NAD(P)H: quinone oxidoreductase C609T polymorphism and the risk of cervical cancer in Japanese subjects. Gynecol Oncol 2005;96:423-9.
- Park SJ, Zhao H, Spitz MR, et al. An association between NQO1 genetic polymorphism and risk of bladder cancer. Mutat Res 2003;536:131-7.
- Wang YH, Lee YH, Tseng PT, et al. Human NAD(P)H:quinone oxidoreductase 1 (NQO1) and sulfotransferase 1A1 (SULT1A1) polymorphisms and urothelial cancer risk in Taiwan. J Cancer Res Clin Oncol 2008;134:203-9.
- Hamajima N, Matsuo K, Iwata H, et al. NAD(P)H: quinone oxidoreductase 1 (NQO1) C609T polymorphism and the risk of eight cancers for Japanese. Int J Clin Oncol 2002;7:103-8.
- Longuemaux S, Deloménie C, Gallou C, et al. Candidate genetic modifiers of individual susceptibility to renal cell carcinoma: a study of polymorphic human xenobiotic-metabolizing enzymes. Cancer Res 1999;59:2903-8.
- Siegelmann-Danieli N, Buetow KH. Significance of genetic variation at the glutathione S-transferase M1 and NAD(P)H:quinone oxidoreductase 1 detoxification genes in breast cancer development. Oncology 2002;62:39-45.
- Akkiz H, Bayram S, Bekar A, et al. No association of NAD(P)H: quinone oxidoreductase 1 (NQO1) C609T polymorphism and risk of hepatocellular carcinoma development in Turkish subjects. Asian Pac J Cancer Prev 2010;11:1051-8.
- Begleiter A, Norman A, Leitao D, et al. Role of NQO1 polymorphisms as risk factors for squamous cell carcinoma of the head and neck. Oral Oncol 2005;41:927-33.
- Kolesar JM, Dahlberg SE, Marsh S, et al. The NQO1*2/*2 polymorphism is associated with poor overall survival in patients following resection of stages II and IIIa non-small cell lung cancer. Oncol Rep 2011;25:1765-72.
- Lafuente MJ, Casterad X, Trias M, et al. NAD(P)H:quinone oxidoreductase-dependent risk for colorectal cancer and its association with the presence of K-ras mutations in tumors. Carcinogenesis 2000;21:1813-9.
- Begleiter A, Hewitt D, Maksymiuk AW, et al. A NAD(P)H:quinone oxidoreductase 1 polymorphism is a risk factor for human colon cancer. Cancer Epidemiol Biomarkers Prev 2006;15:2422-6.
- van der Logt EM, Bergevoet SM, Roelofs HM, et al. Role of epoxide hydrolase, NAD(P)H:quinone oxidoreductase, cytochrome P450 2E1 or alcohol dehydrogenase genotypes in susceptibility to colorectal cancer. Mutat Res 2006;593:39-49.
- Qiu XB, Schönthal AH, Cadenas E. Anticancer quinones induce pRb-preventable G2/M cell cycle arrest and apoptosis. Free Radic Biol Med 1998;24:848-54.
- Xue BJ, Jin ZC. Lochnerol elevates expression of NQO1 and inhibits proliferation of liver cancer cell. Chin J Pathophysiol 2002;18:884-8.
- Begleiter A, Leith MK, Thliveris JA, et al. Dietary induction of NQO1 increases the antitumour activity of mitomycin C in human colon tumours in vivo. Br J Cancer 2004;91:1624-31.


