Circulating tumor cells (CTCs) in breast cancer: a diagnostic tool for prognosis and molecular analysis
Abstract: Metastatic breast cancer (MBC) is characterized by a combination of tumor growth, proliferation and metastatic progression and is typically managed with palliative intent. The benefit of standard systemic therapies is relatively limited and the disease is considered incurable suggesting the need to investigate the biological drivers of the various phases of the metastatic process in order to improve the selection of molecularly driven therapies. The detection, enumeration and molecular analysis of circulating tumor cells (CTCs) provide an intriguing opportunity to advance this knowledge. CTCs enumerated by the Food and Drugs Administration-cleared CellSearch® system are an independent prognostic factor of progressionfree survival (PFS) and overall survival (OS) in MBC patients. Several published papers demonstrated the poor prognosis for MBC patients that presented basal CTC count ≥5 in 7.5 mL of blood. Therefore, the enumeration of CTCs during treatment for MBC provides a tool with the ability to predict progression of disease earlier than standard timing of anatomical assessment using conventional radiological tests. During the metastatic process cancer cells exhibit morphological and phenotypic plasticity undergoing epithelialmesenchymal transition (EMT). This important phenomenon is associated with down regulation of epithelial marker (e.g., EpCAM) with potential limitations in the applicability of current CTCs enrichment methods. Such observations translated in a number of investigations aimed at improving our capabilities to enumerate and perform molecular characterization of CTCs. Theoretically, the phenotypic analysis of CTCs can represent a “liquid” biopsy of breast tumor that is able to identify a new potential target against the metastatic disease and advanced the development and monitoring of personalized therapies.
Key words: Circulating tumor cells (CTCs); metastatic breast cancer (MBC); epithelial-mesenchymal transition (EMT); cancer stem cells
Introduction
With 5.2 million cases, female breast cancer is the most prevalent neoplasm worldwide, with one in six cancer survivors in 2008 diagnosed within the previous 5 years (1). Thus, the high prevalence of the disease in almost all countries of the world indicates the need for improvements in the diagnosis, care and specialized management of the disease. Breast cancer is now recognized to be a heterogeneous disease comprised of several common different phenotypes requiring a more personalized and targeted approach to treatment (2). However, despite optimal local and systemic adjuvant treatment, 30-40% of patients diagnosed in Western Countries with curable breast cancer eventually die of recurrent disease (3). Metastatic disease is the primary cause of cancer death in patients with recurrent disease. The medical management of this condition is typically palliative and include a series of considerations in the selection of the most appropriate and effective treatment (4). These factors typically include: (I) the type and extent of disease and performance status of the patients; (II) biomarkers analysis from tissue specimens of either primary or metastatic sample that will inform about the potential utility of molecularly target therapies; (III) toxicity of standard therapies and availability of clinical trials for the specific condition.
In spite of such sophisticated and accurate evaluation process the majority of treatments are associated with minimal tumor response or benefit and typically a disease progression occur in short period of time. Furthermore, additional biomarkers assessment in patients with refractory conditions is frequently limited by the possibility to perform invasive procedures because of the location of the metastatic disease and the potential for organ damage and complications, therefore an understanding of the various steps of the metastatic process may allow developing tools for risk stratification, biomarkers assessment and therapeutic monitoring. The formation of metastatic colonies is believed to be a continuous process, commencing early during the growth of the primary tumor as occult dissemination (micrometastatic disease) (5). Data on early detection of cancer cells in the bone marrow and lymph nodes as disseminated tumor cells as well as identification of CTCs in the peripheral blood support this view (5,6). The detection and enumeration of CTCs is a particularly attractive clinical modality because it is minimally invasive and can be used to monitor therapy and predict prognosis.
CellSearch™ system for CTC prognosis and monitoring in MBC
The detection and characterization of circulating tumor cells represent a potential area of technology development that can demonstrate such significant clinical utility in the determination of the initial biomarkers expression and subsequent therapeutic monitoring. CTCs are very rare cancer cells surrounded by billion of hematopoetic cells in the bloodstream. To identify CTCs in the peripheral blood, CTCs should be distinguished from normal hematopoetic cells as well as from normal epithelial cells (7). Different strategies are used to reach this goal including morphological and physical characteristics like size and weight, or expression of specific markers (8,9). In carcinomas, which are tumors of epithelial origin, CTCs are usually identified based on the expression of epithelial lineage markers likes cytokeratins (cytoskeletal proteins present in epithelial cells) or EpCAM (epithelial cell adhesion molecule), absence of common leukocyte marker (CD45) and/or by putative tumor-specific antigens (MUC1, HER-2 and/or others) (10). Due to rarity of CTCs, enrichment steps are usually necessary to increase the detection success rate. Most common enrichment strategies exploit immunomagnetic or other immunoseparation techniques. Typically, antibody specific to EpCAM is conjugated with magnetic particles, and epithelial cells tagged to antibody are separated in a magnetic field. Given the CTCs rarity, our biological and clinical insights into CTCs are strongly dependent on the parameters of the isolation technologies (11,12). Despite recent technological advances, we are still in the beginning in the understanding of the processes played by CTCs in tumor dissemination and progression.
The majority of data on the clinical utility of CTCs derived from studies using the the FDA-approved CellSearch™ technology (Veridex, LLC, NJ, US) that demonstrated the prognostic value in patients with various forms of solid tumors, including breast, prostate and colon cancer (13-15). The baseline prognostic value of CTCs proved particularly useful in patients with newly diagnosed metastatic breast cancer (MBC) prior to beginning first-line salvage therapy as demonstrated in a series of studies. The first large, multi-institution, double-blind, prospective clinical trial evaluated the prognostic capability of CTCs in patients with MBC (13). One hundred and seventy-seven patients with measurable disease had CTCs tested prior to beginning a new palliative treatment regimen for progressive disease, followed by repeat assessment at first follow-up visit approximately 4 weeks later. This landmark trial prospectively identified a CTCs cut-off level of ≥5 cells per 7.5 mL of blood to be a reliable identifier of patients at higher risk for disease progression and decreased survival from metastatic breast cancer. Regardless of histology, hormone receptor and HER2/neu status, or whether the patient had recurrent or de novo metastatic disease, those with <5 CTCs at baseline, and more importantly, at first follow-up after beginning a new treatment regimen, had superior progression free (PFS) and overall survival (OS) [7 vs. 2.1 months (P<0.001) and 10.1 vs. >18 months, (P<0.001), respectively]. Additional CTCs assessment in this same patient cohort at essentially monthly intervals following first follow-up also confirmed improved PFS and OS for patients with <5 CTCs at continued interval testing. PFS and OS for patients with <5 CTCs ranged from 5.6 to 7 months and 18.6 to >25.0 months, respectively, compared to those with ≥5 CTCs of 1.3 to 3.6 months PFS and 6.3 to 10.9 months OS (P=0.001) at anytime throughout the 6 months observational period (15). These data were subsequently confirmed by various authors and expanded the observation of the clinical utility of CTCs (16-21). Liu and colleagues recently conducted a pooled analysis of a number of clinical studies performed in MBC using the CellSearch™ technology and testing patients at baseline and follow-up during the course of the disease. Six studies were identified enrolling a total of 841 patients worldwide (22). The detection of ≥5 CTCs was confirmed associated with prognostic significance at baseline and subsequent monitoring. The prognostic value was independent of disease subtype and type of therapy and in multivariate analysis demonstrated the strongest factor associated with survival. The management of breast cancer evolved in the last decade with the increased understanding that various breast cancer subtypes differ in their baseline prognosis, response to chemotherapy and benefit to molecularly targeted therapies (23,24). Those important data suggested the need to further explore the prognostic value of CTCs in relation to those factors (disease subtype and type of therapy) (25-27). Munzone and colleagues performed a retrospective analysis of 203 consecutive patients with metastatic breast cancer with baseline CTC enumeration. Patients were categorized into 3 prognostic groups based on the number of CTCs (0, 1-4, and ≥5) and into 5 categories based on tumor biological characteristics: luminal-A {estrogen receptor [ER] and progesterone receptor [PR] >1%, grade 1/2, human epidermal growth factor 2 [HER2]-negative [HER2(-)], Ki67 value <14%}; luminal-B [ER and/or PR >1%, grade 3, HER2(-), Ki67 value >14%]; luminal-B HER2-positive [HER2(+)] [ER and/or PR >1%, any grade, HER2(+), Ki-67 value any]; HER2(+) [HER2 overexpressed/fluorescence in situ hybridization (FISH) amplified, ER and PR absent]; triple negative (TN) (ER and PR 0%, HER2 not overexpressed/FISH not amplified). Twenty-seven patients (13.3%) had luminal-A category, 105 (51.7%) patients had luminal-B, 29 (14.3%) patients had luminal-B HER2(+), 24 patients (11.8%) had HER2(+), and 18 patients (8.9%) had triple negative disease. CTCs were mostly found in patients with luminal-A/luminal-B HER2(-) subtype. At multivariable analysis, CTC count was a significant predictive factor for OS in all molecular subtypes (log-rank P<0.01). Giordano et al., reported on a retrospective analysis of 517 patients treated at the University of Texas, M D Anderson Cancer Center. Patients were categorized in 4 subtypes; luminal A (56.4%), luminal B (9.7%), HER-2 disease (9.9%) and TN disease (24%). Two hundred and six (40%) patients had ≥5 CTCs at baseline blood draw, and 311 (60%) had <5 CTCs. A larger proportion of luminal A patients had ≥5 CTCs than did patients with other subtypes of tumor (P=0.024). With regards to treatment administer, chemotherapy alone, chemotherapy plus bevacizumab, anti-HER2 combination treatment, hormonal treatment, or other investigational treatments were administered in 48%, 13%, 15%, and 19% or 5% of cases, respectively. At a median follow-up period of 24.6 months, 456 (88%) of 517 patients had showed progression of disease and 276 (53%) patients had died. The median OS and PFS were significantly different in luminal A patients (n=292) with ≥5 CTCs than in patients with <5 CTCs (OS, 18.8 versus 48.7 months, P<0.001; and PFS, 5.9 versus 7.1, P=0.004). In luminal B patients with ≥5 CTCs, the median OS was 29.5 months versus not yet reached in patients with <5 CTCs (P=0.084). Moreover, among TN breast cancers (n=124), patients with ≥5 CTCs had a median OS significantly shorter than patients with <5 CTCs (10.4 versus 17.8 respectively, P=0.001). Median PFS was similar for TN breast cancer patients with ≥5 CTCs and patients with <5 CTCs (PFS, 5.1 versus 4.8, respectively, P=0.274). Instead, the hazard ratio of death in patients with ≥5 CTCs who had undergone anti-HER2-targeted therapy did not significantly differ from that of patients with <5 CTCs, suggesting an interaction between those therapies and CTCs detected by the CellSearch™ method. Interestingly, similar results were demonstrated for patients receiving first-line treatment with bevacizumab (Avastin®)-based regimens. These studies seem to suggest that therapeutic monitoring of patients with MBC may benefit from integration of other CTCs detection methods to be used along with the CellSearch™.
The capacity of predicting outcome at multiple time points raised interesting observation about the potential use of CTCs monitoring as complementary modality to standard imaging, particularly when evaluating benefit and detecting progression of disease. This approach could prove particularly useful when evaluating disease sites that are not measurable but only evaluable as bone metastases and in situations with more indolent disease. Oxnard et al. recently reported on the issue of appropriate disease evaluation in patients with advanced cancer suggesting that response and progression play two very different roles in solid tumor oncology and the two may be better conceptualized as distinct events rather than the two ends of a single spectrum (28). In these regards, particularly challenging is the demonstration of progression of disease using a combination of modalities including clinical assessment for measurement of symptoms, imaging and serum markers. In order to more accurately evaluate the role of CTCs in these scenarios we conducted a series of problem-focused analysis (29-33). In a single Institution, retrospective study including 185 patients diagnosed between 2001 and 2007 patients with ≥5 CTCs had a greater than three-and-a-half fold greater hazard of death, [HR=3.64 (95% CI, 2.11-6.30, P<0.0001)] compared to those patients with <5 CTCs at baseline (29). The prognostic significance of CTCs precluded choice of therapy (i.e., - chemotherapy with anthracyclines, taxanes, or both anthracyclines/taxanes, hormone therapy), and was also independent of hormone receptor status and HER-2/neu status. Interestingly, in this cohort, although the patient demographics were representative of the phenotypic characteristics of MBC patients in general, i.e., - approximately two-thirds of patients hormone receptor positive and approximately 20% HER-2/neu positive, greater than half of the patients had bone as their first site of metastatic disease. Upon multivariate analysis, patients with bone metastasis, compared to other sites of disease with >5 CTCs had an additional risk of death [HR=1.61; 95% CI, 0.52-5.04 (P=0.410)]. These interesting findings suggested that CTCs enumeration can be used to monitor treatments along with standard imaging modalities. Furthermore, Budd et al. compared the CTCs monitoring to bidimensional response assessment (central review) of 177 patients with MBC (30). Remarkably, the determination of CTCs at baseline and follow-up appeared to have superior prognostic implications in patients with measurable MBC compared to standard imaging assessment, particularly in patients with more refractory disease. Subsequently, De Giorgi et al. completed a retrospective analyses of 115 MBC patients who started a new line of therapy and who had CTC counts and fluoro-deoxyglucose (FDG)-Positron Emission Tomography (PET) scans performed at baseline and at 9 to 12 weeks during therapy (midtherapy) (31). In 102 evaluable patients, the median overall survival time was 14 months (range, 1 to >41 months). Midtherapy CTC levels correlated with FDG-PET/CT response in 68 (67%) evaluable patients. In univariate analysis, midtherapy CTC counts and FDG-PET/CT response predicted overall survival (P<0.001 and P=0.001, respectively). FDG-PET/CT predicted overall survival (P=0.0086) in 31 (91%) of 34 discordant patients who had fewer than five CTCs at midtherapy. Only midtherapy CTC levels remained significant in a multivariate analysis (P=0.004) further supporting the critical importance of this test in the management of MBC in comparison with sensitive but, quite expensive functional imaging. The correlation between these different monitoring modalities in advanced disease was further refined in a series subsequent analysis from the same team of investigators with particular regards to patients with bone metastases (32,33). The largest study evaluated 195 patients with MBC who were diagnosed with relapsed/progressive MBC underwent FDG-PET/CT scans and provided blood samples for CTC analysis. One hundred seventeen (60%) patients had received prior treatment of MBC with hormonal therapy (53 cases), chemotherapy with or without hormonal therapy (48 cases), or human epidermal growth factor receptor 2 (HER2)-targeted therapies combined with chemotherapy and/or hormonal therapy (16 cases), whereas 78 (40%) had new diagnoses with MBC. Interestingly, the analysis demonstrated that among the 137 patients with bone metastases at relapse/progression, 83 (61%) had ≥5 CTCs, while 54 (39%) had <5 CTCs (P=0.0122). Higher CTC numbers were detected in patients with bone metastases alone and patients with metastases in bone plus other sites relative to those with no bone metastases. Moreover, higher CTC numbers were detected in the patients with more extensive bone metastases relative to those with one or two bone lesions. With regards to the correlation with imaging findings, all 137 patients with bone metastases but seven had increased FDG uptake within one or more lesions. Of these seven cases, four had <5 CTCs; of the remaining three with ≥5 CTCs, two also had liver metastases with elevated FDG uptake (CTCs =143 and 25), while one presented with primary tumor with elevated FDG uptake (CTCs =75). This represented a significant association between CTCs detection and bone metastases and supported the use of PET/CT as imaging modality for evaluation of patients with this predominance in this disease site. In summary, CTCs enumeration at baseline and follow-up demonstrated strong association with prognosis and proved useful in therapeutic monitoring suggesting a complementary role with current standard imaging modalities.
Novel CTC detection methods
The CellSearch™ method detect CTCs in approximately 60% of patients with advanced breast, prostate and colon cancer somewhat limiting the widespread utilization of such testing for therapeutic monitoring. In the last few years investigations in the molecular bases of the metastatic process have provide insights to clarify the molecular heterogeneity of CTCs (34). A number of studies have shown that carcinoma cells often activate a transdifferentiation program, termed epithelial-mesenchymal transition (EMT) (35,36). These cells acquire the traits needed to execute multiple steps of metastasis. Through this EMT process, epithelial cells lose cell-cell contacts and cell polarity, downregulate epithelial-associated genes, acquire mesenchymal gene expression, and undergo major changes in their cytoskeleton (36). This cellular process culminates in a mesenchymal appearance with increased motility and invasiveness.
Mani et al. showed that induction of EMT in immortalized human mammary epithelial cells results in de novo expression of stem cell markers and acquisition of functional stem cell properties, including the ability to form mammospheres (37). These findings illustrated a link between the EMT process and cancer stem cells and suggest that EMT contributes to the heterogeneity of tumor-initiating potential observed amongst breast tumor cells. It is supposed that EMT plays a major role in the initial step of the metastatic cascade, where, through EMT, some of the tumor cells acquire the ability to invade the basement membrane and surrounding stroma and then intravasate (38). Following extravasation, a process termed mesenchymal to epithelial transition (MET) has been proposed. It is proposed that tumor cells in the secondary organ undergo “redifferentiation” into an epithelial phenotype and thus form metastases with similar histological characteristics as the primary tumor. It is still unclear if all disseminated cancer cells undergo MET and lose their mesenchymal/cancer stem cell phenotype or if the fraction of mesenchymal/cancer stem cells give rise to numerous differentiated progeny during colonization (36,38). However, both cases would result in a differentiated, epithelial tumor phenotype. There experimental or translational data linking EMT and CTCs.
In an experimental model, blocking the expression of the EMT inducing transcription factor, TWIST, in the highly metastatic 4T1 murine mammary cell line reduced both metastatic burden and the number of CTCs in mice bearing xenograft mammary tumors (39). In another model, hamster oral keratinocytes were transformed by downstream effectors of the TGF beta pathway (p12CKD2-AP1) and exhibit EMT phenotype (40). After subcutaneous injection in mice both control as well as EMT induced cells were competent to form tumors, but only EMT induced cells were detected in the bloodstream. However, neither cells could form lung metastases. In contrast, after intravenous inoculation only non-EMT cells developed lung metastasis. Similarly, Chaffer et al. comparing bladder cancer cell lines showed that parenteral cell line with EMT characteristics was able to form lung metastasis after injection in mouse bladder in contrast to more epithelial daughter cell line isolated from bone metastasis via in vivo selection (41). Aktas et al. evaluated expression of EMT-associated markers on CTCs (TWIST1, AKT2, PI3Kalpha) using an RT-PCR based assay that includes a pre-enrichment for blood-borne cells expressing a common epithelial antigen, i.e., EpCAM (42). This study found that 62% of the CTCs were positive for at least one of these EMT markers. In other studies, it was shown that phosphorylated EGFR, HIF1 alfa, HER2 and PI3K/Akt signaling kinases are expressed in CTCs and it is known that these pathways can regulate EMT (42). However, these studies used epithelial markers for detection of CTCs. Therefore, we can hypothesize that some of the CTCs in these studies had a partial EMT phenotype.
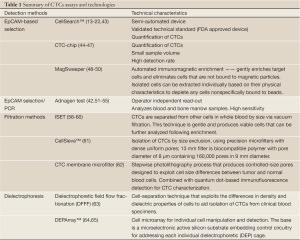
In summary, while the CellSearch™ method demonstrated clinical value in epithelial malignancies, the heterogeneity of CTCs based on the acquisition of EMT phenotype during the metastatic process requires novel methods that will be have to rely on other selection methods and not necessarily on epithelial enrichment (Table 1). We describe the properties of number of novel CTC detection methods developed in the last few years.
Full table
CTC-Chip: a novel “CTC-Chip” represents microchip technology on a microfluidic platform, optimized for the isolation of CTCs (44,45). The tool enables interaction between CTCs and microposts coated with an antibody against EpCAM under precisely controlled laminar-flow conditions (44). As CTC detection is EpCam-based, the same promises and limitations apply as described above for EpCAM-based immunomagnetic separation. In pilot study, the CTC-chip revealed presence of cytokeratin positive CTCs in nearly all (i.e., 115 of 116 analyzed) patients, yet even differential analysis of CTC numbers revealed no association with tumor stage (44). In a first clinical and more promising approach, the chip had been applied to NSCLC patient samples, demonstrating the feasibility for CTC monitoring and assessment of genetic marker guided treatment effects (46). More recently the same group introduced the herringbone-chip, or “HB-Chip”, an improved microfluidic platform (46,47). Efficient cell capture was validated using defined numbers of cancer cells spiked into control blood, and clinical utility was demonstrated in specimens from patients with prostate cancer (47). CTCs were detected in 14 of 15 (93%) patients with metastatic disease (median =63 CTCs/mL, mean = 386±238 CTCs/mL), and the tumor-specific TMPRSS2-ERG translocation was readily identified following RNA isolation and RT-PCR analysis.
Adnagen: another advancement in the field is the AdnaTest (Alere, San Diego, CA, US). The AdnaTest can be performed on standard technology platforms. AdnaGen’s two-step ‘Combination-of-Combinations Principle’ (51,52). It initially involves cell isolation, whereby tumor cells are enriched by an antibody-mix (EpCAM, MUC1) linked to magnetic particles and mRNA is isolated from the selected tumor cells, and subsequent molecular biological detection and analysis, whereby the isolated mRNA is transcribed into cDNA and a multiplex PCR is carried out for the analysis of tumor-associated gene expression (51). The AdnaTest has a reported analytical sensitivity of two CTCs per 5 mL of blood. Andreopoulou and colleagues compared this method with the CellSearch® in 55 patients with MBC (53). AdnaTest was positive in 29 (53%) with the individual markers being positive in 18% (GA733-2), 44% (MUC-1), and 35% (HER2). 26 (47%) patients were positive for CTCs by the CellSearch (≥2 cutoff), while 20 (36%) were positive (≥5 cutoff). Overall positive agreement was 73% for CTC≥2 and 69% for CTC≥5. Interestingly, the AdnaTest detected HER2 expression in CTCs in 19 patients of which, HER2 status of the primary tumor was positive (HER-2 overexpressed or amplified) and concordant in only five patients (26%), negative and discordant in 11 (58%), and unknown in three of these patients, supporting the concept of tumor heterogeneity. Aktas and colleagues using the same technology in patients with early breast cancer were able to demonstrate a difference in the expression level of ER between primary tumor and CTCs (43). In particular, the peripheral cancer cells appeared either lacking the expression of ER in the majority of cases in spite of hormone-receptor positive disease (54). The study confirmed the higher level of HER-2 expression in CTCs. Most recently, the technology allowed for identification of cancer stem cells using the AdnaTest EMT-1/Stem CellCellect and Detect that identify cells expressing TWIST1, Akt2, PI3Kα and ALDH-1 (55). Kasimir-Bauer showed that patients with resistance to standard chemotherapy regimens have detectable CTCs with characteristics of CSCs. These data has potential implications with regards to the the understanding of the bases of endocrine resistance (e.g., lack of ER expression in CTCs). A prospective clinical study will evaluate the prognostic and predictive value of the Adnagen method in MBC patients receiving endocrine therapy.
MagSweeper: this enrichment technology is based on EpCAM immunomagnetic separation that gently purifies rare cells present in a mixed population. The final purity of isolated target cells in immunomagnetic-based separation devices depends first on the specificity of the antibodies used to select the desired cells and second on the amount of nonspecific cell capture (48). Initial studies demonstrated the capacity to recover epitelial cells with for expression profiling analysis and with increased tumorigenic properties (48,49). Powell and colleagues used the MagSweeper to isolate alive cells from patients with primary (14/20) and metastatic breast cancer. In the same prospective study they showed no recovery of epithelial cells in patients with non-epithelial cancer (n=20) or healthy subjects (n=25) (50). Subsequently, single cell transcriptional profiling of 87 cancer-associated and reference genes was performed and showed heterogeneity among individual CTCs, separating them into two major subgroups, based on 31 highly expressed genes. These investigators completed a concomitant analysis of single cells from seven breast cancer cell lines showing a much more clustering, mostly by ER status. Elevated transcript levels of genes associated with metastasis NPTN, S100A4, S100A9, and with epithelial mesenchymal transition: VIM, TGFß1, ZEB2, FOXC1, CXCR4, were striking findings in CTCs compared to cell lines. They concluded that CTC profiles were distinct from those of cancer cell lines and a more appropriate reflection of the metastatic process. The data raise questions on the suitability of cell lines for drug discovery efforts for late stage cancer therapy and making CTCs a more appropriate model.
Isolation by size of epithelial tumor cells [ISET]: the ISET is enrichment-free filtration method based on the assumption of uniform larger size of cancer cells compared to peripheral blood leukocytes. The system is constitute of a polycarbonate Track-Etch-type membrane with calibrated, 8-µm-diameter, cylindrical pores (56,57). The module of filtration has 12 wells, making it possible to load and filter 12 individual samples in parallel. Each sample is filtered through a 0.6-cm-diameter surface area in the membrane. Ten milliters of diluted solution, corresponding to 1 mL of undiluted blood, is loaded on each well and filtered by gentle aspiration under vacuum (created by a vacuum pump). Individual CTCs are made available for morphological, immunocytological, and genetic characterization. This method proved the ability to detect cells in early breast cancer, melanoma and non-small cell lung cancer (NSCLC) (58-60). Prospective application for biomarkers evaluation and therapeutic monitoring are currently ongoing.
DEPArray: the DEPArray™ technology is a new separation method based on the unique dielectrophoretic properties of cancer cells (63,64,66). This microelectronic active silicon substrate embedding control circuitry is applied sequentially to specimens that have undergone initial epithelial-base enrichment (e.g., CellSearch® selection) but still contaminated with mononuclear cells that could affect the recovery of quality DNA and mRNA for molecular analysis (64). Preliminary experience demonstrated the capacity to address tumor morphology and heterogeneity in patients with different disease subtype in MBC (Figures 1,2). Moreover, the collection of pure cancer cells allow the possibility of using the Single-Cell Ampli1 WGA for DNA amplification and detection of P53 mutations in CTCs and compare with similar analysis in metastatic disease (65). This approach holds great promise for molecular characterization of single cells, evaluate tumor heterogeneity and implement monitoring of personalized therapies.
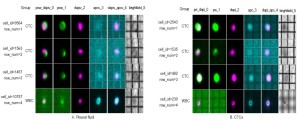
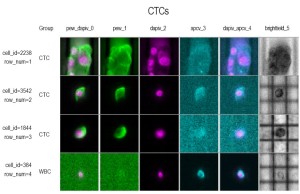
Conclusions
The detection of CTCs in the peripheral blood of patients with MBC is associated with prognostic and predictive information allowing for baseline risk stratification, therapeutic monitoring and assessment of metastatic progression. Improvements in enrichment and selection methods are associated with an increase in the capability to capture a higher number of cancer cells and perform molecular characterization. CTCs offer the intriguing possibility to explore the “liquid phase” of solid tumors, an area of investigation unexplored for many years. This new concept suggests a new approach to the management of MBC patients including a “real-time” assessment of biomarkers for selection and monitoring of targeted therapies with the goal of implementing more effective personalized therapies.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Bray F, Ren JS, Masuyer E, et al. Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer 2012. [Epub ahead of print].
- Sørlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A 2001;98:10869-74.
- Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277-300.
- Cardoso F, Costa A, Norton L, et al. 1st International consensus guidelines for advanced breast cancer (ABC 1). Breast 2012;21:242-52.
- Braun S, Vogl FD, Naume B, et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med 2005;353:793-802.
- Racila E, Euhus D, Weiss AJ, et al. Detection and characterization of carcinoma cells in the blood. Proc Natl Acad Sci U S A 1998;95:4589-94.
- Gross HJ, Verwer B, Houck D, et al. Model study detecting breast cancer cells in peripheral blood mononuclear cells at frequencies as low as 10(-7). Proc Natl Acad Sci U S A 1995;92:537-41.
- Hawes D, Neville AM, Cote RJ. Occult metastasis. Biomed Pharmacother 2001;55:229-42.
- Hu XC, Chow LW. Detection of circulating breast cancer cells with multiple-marker RT-PCR assay. Anticancer Res 2001;21:421-4.
- Witzig TE, Bossy B, Kimlinger T, et al. Detection of circulating cytokeratin-positive cells in the blood of breast cancer patients using immunomagnetic enrichment and digital microscopy. Clin Cancer Res 2002;8:1085-91.
- Kagan M, Howard D, Bendele T, et al. A sample preparation and analysis system for identification of circulating tumor cells. J Clin Ligand Assay 2002;25:104-10.
- Terstappen LW, Rao C, Gross S, et al. Peripheral blood tumor cell load reflects the clinical activity of the disease in patients with carcinoma of the breast. Int J Oncol 2000;17:573-8.
- Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 2004; 351:781-91.
- de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res 2008;14:6302-9.
- Cohen SJ, Punt CJ, Iannotti N, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol 2008;26:3213-21.
- Cristofanilli M, Hayes DF, Budd GT, et al. Circulating tumor cells: A novel prognostic factor for newly diagnosed metastatic breast cancer. J Clin Oncol 2005;23:1420-30.
- Cristofanilli M, Broglio KR, Guarneri V, et al. Circulating tumor cells in metastatic breast cancer: Biologic staging beyond tumor burden. Clin Breast Cancer 2007;7:471-9.
- Nolé F, Munzone E, Zorzino L, et al. Variation of circulating tumor cell levels during treatment of metastatic breast cancer: prognostic and therapeutic implications. Ann Oncol 2008;19:891-7.
- Liu MC, Shields PG, Warren RD, et al. Circulating tumor cells: a useful predictor of treatment efficacy in metastatic breast cancer. J Clin Oncol 2009;27:5153-9.
- Nakamura S, Yagata H, Ohno S, et al. Multi-center study evaluating circulating tumor cells as a surrogate for response to treatment and overall survival in metastatic breast cancer. Breast Cancer 2010;17:199-204.
- Pierga JY, Hajage D, Bachelot T, et al. High independent prognostic and predictive value of circulating tumor cells compared with serum tumor markers in a large prospective trial in first-line chemotherapy for metastatic breast cancer patients. Ann Oncol 2012;23:618-24.
- Liu MC, Mego M, Nakamura S, et al. Clinical validity of circulating tumor cell (CTC) enumeration in 841 subjects with metastatic breast cancer (MBC). J Clin Oncol 2011;29:abstr 10592.
- Sørlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A 2001;98:10869-74.
- Esserman LJ, Berry DA, Demichele A, et al. Pathologic Complete Response Predicts Recurrence-Free Survival More Effectively by Cancer Subset: Results From the I-SPY 1 TRIAL--CALGB 150007/150012, ACRIN 6657. J Clin Oncol 2012;30:3242-9.
- Giordano A, Giuliano M, De Laurentiis M, et al. Circulating tumor cells in immunohistochemical subtypes of metastatic breast cancer: lack of prediction in HER2-positive disease treated with targeted therapy. Ann Oncol 2012;23:1144-50.
- Munzone E, Botteri E, Sandri MT, et al. Prognostic value of circulating tumor cells according to immunohistochemically defined molecular subtypes in advanced breast cancer. Clin Breast Cancer 2012;12:340-6.
- Bidard FC, Mathiot C, Degeorges A, et al. Clinical value of circulating endothelial cells and circulating tumor cells in metastatic breast cancer patients treated first line with bevacizumab and chemotherapy. Ann Oncol 2010;21:1765-71.
- Oxnard GR, Morris MJ, Hodi FS, et al. When progressive disease does not mean treatment failure: reconsidering the criteria for progression. J Natl Cancer Inst 2012;104:1534-41.
- Dawood S, Broglio K, Valero V, et al. Circulating tumor cells in metastatic breast cancer: From prognostic stratification to modification of the staging system? Cancer 2008;113:2422-30.
- Budd GT, Cristofanilli M, Ellis MJ, et al. Circulating tumor cells versus imaging: Predicting overall survival in metastatic breast cancer. Clin Cancer Res 2006;12:6403-9.
- De Giorgi U, Valero V, Rohren E, et al. Circulating tumor cells and [18F] fluorodeoxyglucose positron emission tomography/computed tomography for outcome prediction in metastatic breast cancer. J Clin Oncol 2009;27:3303-11.
- De Giorgi U, Mego M, Rohren EM, et al. 18F-FDG-PET/CT findings and circulating tumor cell counts in the monitoring of systemic therapies for bone metastases from breast cancer. J Nucl Med 2010;51:1213-8.
- Mego M, De Giorgi U, Dawood S, et al. 18F-FDG PET/CT findings and circulating tumor cell counts in the monitoring of systemic therapies for bone metastases from breast cancer. Int J Cancer 2011;129:417-23.
- Powell AA, Talasaz AH, Zhang H, et al. Single cell profiling of circulating tumor cells: transcriptional heterogeneity and diversity from breast cancer cell lines. PLoS One 2012;7:e33788.
- Thiery JP. Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol 2003;15:740-6.
- Hay ED. An overview of epithelio-mesenchymal transformation. Acta Anat (Basel) 1995;154:8-20.
- Mani SA, Guo W, Liao MJ, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008;133:704-15.
- Hollier BG, Evans K, Mani SA. The epithelial-to-mesenchymal transition and cancer stem cells: a coalition against cancer therapies. J Mammary Gland Biol Neoplasia 2009;14:29-43.
- Yang J, Mani SA, Donaher JL, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 2004;117:927-39.
- Tsuji T, Ibaragi S, Shima K, et al. Epithelial-mesenchymal transition induced by growth suppressor p12CDK2-AP1 promotes tumor cell local invasion but suppresses distant colony growth. Cancer Res 2008;68:10377-86.
- Chaffer CL, Brennan JP, Slavin JL, et al. Mesenchymal-to-epithelial transition facilitates bladder cancer metastasis: role of fibroblast growth factor receptor-2. Cancer Res 2006;66:11271-8.
- Aktas B, Tewes M, Fehm T, et al. Stem cell and epithelial-mesenchymal transition markers are frequently overexpressed in circulating tumor cells of metastatic breast cancer patients. Breast Cancer Res 2009;11:R46.
- Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 2004;351:781-91.
- Nagrath S, Sequist LV, Maheswaran S, et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 2007;450:1235-9.
- Maheswaran S, Sequist LV, Nagrath S, et al. Detection of mutations in EGFR in circulating lung-cancer cells. N Engl J Med 2008;359:366-77.
- Stott SL, Lee RJ, Nagrath S, et al. Isolation and characterization of circulating tumor cells from patients with localized and metastatic prostate cancer. Sci Transl Med 2010;2:25ra23.
- Stott SL, Hsu CH, Tsukrov DI, et al. Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proc Natl Acad Sci U S A 2010;107:18392-7.
- Talasaz AH, Powell AA, Huber DE, et al. Isolating highly enriched populations of circulating epithelial cells and other rare cells from blood using a magnetic sweeper device. Proc Natl Acad Sci U S A 2009;106:3970-5.
- Ameri K, Luong R, Zhang H, et al. Circulating tumour cells demonstrate an altered response to hypoxia and an aggressive phenotype. Br J Cancer 2010;102:561-9.
- Powell AA, Talasaz AH, Zhang H, et al. Single cell profiling of circulating tumor cells: transcriptional heterogeneity and diversity from breast cancer cell lines. PLoS One 2012;7:e33788.
- Zieglschmid V, Hollmann C, Gutierrez B, et al. Combination of immunomagnetic enrichment with multiplex RT-PCR analysis for the detection of disseminated tumor cells. Anticancer Res 2005;25:1803-10.
- Zieglschmid V, Hollmann C, Böcher O. Detection of disseminated tumor cells in peripheral blood. Crit Rev Clin Lab Sci 2005;42:155-96.
- Andreopoulou E, Yang LY, Rangel KM, et al. Comparison of assay methods for detection of circulating tumor cells in metastatic breast cancer: AdnaGen AdnaTest BreastCancer Select/Detect™ versus Veridex CellSearch™ system. Int J Cancer 2012;130:1590-7.
- Aktas B, Müller V, Tewes M, et al. Comparison of estrogen and progesterone receptor status of circulating tumor cells and the primary tumor in metastatic breast cancer patients. Gynecol Oncol 2011;122:356-60.
- Kasimir-Bauer S, Hoffmann O, Wallwiener D, et al. Expression of stem cell and epithelial-mesenchymal transition markers in primary breast cancer patients with circulating tumor cells. Breast Cancer Res 2012;14:R15.
- Rostagno P, Moll J, Bisconte J, et al. Detection of rare circulating breast cancer cells by filtration cytometry and identification by DNA content: sensitivity in an experimental model. Anticancer Res 1997;17:2481-5.
- Vona G, Sabile A, Louha M, et al. Isolation by size of epithelial tumor cells: a new method for the immunomorphological and molecular characterization of circulating tumor cells. Am J Pathol 2000;156:57-63.
- Pinzani P, Salvadori B, Simi L, et al. Isolation by size of epithelial tumor cells in peripheral blood of patients with breast cancer: correlation with real-time reverse transcriptase-polymerase chain reaction results and feasibility of molecular analysis by laser microdissection. Hum Pathol 2006;37:711-8.
- De Giorgi V, Pinzani P, Salvianti F, et al. Application of a filtration- and isolation-by-size technique for the detection of circulating tumor cells in cutaneous melanoma. J Invest Dermatol 2010;130:2440-7.
- Hofman V, Ilie MI, Long E, et al. Detection of circulating tumor cells as a prognostic factor in patients undergoing radical surgery for non-small-cell lung carcinoma: comparison of the efficacy of the CellSearch Assay™ and the isolation by size of epithelial tumor cell method. Int J Cancer 2011;129:1651-60.
- Adams D, Makarova O, Zu P, et al. Isolation of Circulating Tumor Cells by Size Exclusion using Lithography Fabricated Precision Microfilters. Proceedings 102nd AACR meeting 2011, Orlando, FL, 2011:Abstr 2369.
- Lin HK, Zheng S, Williams AJ, et al. Portable filter-based microdevice for detection and characterization of circulating tumor cells. Clin Cancer Res 2010;16:5011-8.
- Das CM, Becker F, Vernon S, et al. Dielectrophoretic segregation of different human cell types on microscope slides. Anal Chem 2005;77:2708-19.
- Medoro G, Gross S, Manaresi N, et al. Use of the DEPArray platform to detect, isolate, and molecularly characterize pure tumor cells from peripheral blood samples enriched using the CellSearch system. J Clin Oncol 2011;29:abstr 10616.
- Alpaugh KR, Bingham C, Fittipaldi P, et al. Mutational analysis of tissue and circulating tumor cells (CTCs) in advanced inflammatory breast cancer (IBC) reveals tumor heterogeneity. Implications for selection of molecular therapies? Cancer Res 2012; 72 (Suppl).
- Cristofanilli M, De Gasperis G, Zhang L, et al. Automated electrorotation to reveal dielectric variations related to HER-2/neu overexpression in MCF-7 sublines. Clin Cancer Res 2002;8:615-9.


