A case of rapid growing colonic NK/T cell lymphoma complicated by Crohn’s disease
Introduction
Primary gastrointestinal lymphoma accounts for 4% to 20% of all non-Hodgkin’s lymphoma (NHL) cases, which mainly are B cell origin. The primary NK/T cell lymphoma of the intestine is extremely rare, and it comprises 5.2% to 14.7% of all primary intestinal lymphomas (1,2). Due to its nonspecific clinical and endoscopic findings, the early diagnosis of this type of lymphoma is frequently difficult, so the appropriate treatment may be delayed. Herein, we report a case of colonic NK/T cell lymphoma complicated by colonic Crohn’s disease (CD) to investigate the clinicopathological features and immunophenotype of primary colonic NK/T cell lymphoma and its association with CD.
Case report
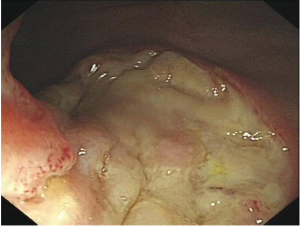
A 37-year-old man with complaints of abdominal pain and bloody diarrhea over a 11 months period was admitted to West China hospital. In his past history, the patient experienced pulmonary tuberculosis for 3 times at ages of 20, 24 and 34 years, respectively. Neither enlarged lymph nodes nor hepatomegaly and splenomegaly were found by physical examination. Enlargement of lymph nodes were not observed in chest and pelvic cavity by color Doppler ultrasonography. Abdominal computed tomography (CT) scan showed pseudopolyps in the initial segment of the ascending colon without evidence of intraabdominal lymph node enlargement. Total colonoscopy at admission revealed multifocal irregular ulcers distributing circumferentially or transversely in his ileocecum, ascending and descending colon (Figure 1). The histological findings of the specimens showed severe active chronic colitis. Laboratory studies showed moderate anemia with hemoglobin concentration of 67 g/L, 32 mm/h for erythrocyte sedimentation rate and 66.5 mg/L for C-reactive protein. Although acid fast stain of colonic biopsies was negative for acid fast bacilli, we made a clinical suspected diagnosis of intestinal tuberculosis (ITB). The diagnostic anti-tuberculosis therapy was then initiated with relief of symptoms.
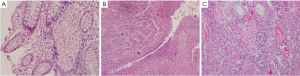
Two months later, however, the patient complained of repeated fever, bloody diarrhea and right lower abdominal pain. The subsequent colonoscopy revealed more irregular ulcers from the ileocecum to descending colon than before. The histological findings showed an extensive suppurative colitis accompanied by focal necrosis. He was clinically suspected with colonic malignant lymphoma, so a subtotal colon resection was performed. The histology of the operative biopsy specimens was featured by transmural inflammation, noncaseating granuloma in the mucoderm (Figure 2A) and deeply situated fissure ulcer reaching the muscularis propria (Figure 2B). Ganglionitis, angiectasia and lymphangiectasia were obviously found in the submucosa (Figure 2C). Moreover, nodular lymphoid aggregates from the submucosa to subserosa and segmental thickening of muscularis propria were observed. The immunophenotypic examination for malignant lymphomas was negative. Based on these findings, the patient was histologically suggested CD, and mesalazine was administered 0.5 g orally three times daily as maintenance therapy postoperatively with improvement of symptoms, then he was discharged.
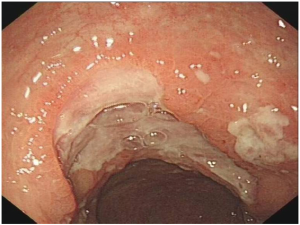
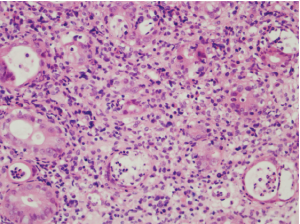
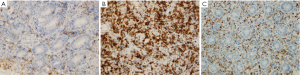
Another two months later, the patient was readmitted because of repeated fever. The colonoscopy performed at readmission showed scattered superficial ulcers along the ileocolon stomas, and mesalazine was then replaced by prednisone tapers (5 to 40 mg daily for 2 months) along with azathioprine (75 mg daily). However, his symptom persisted. Total colonoscopy performed 5 months later revealed an irregular ulcer in the ileocolon stomas up to 4 cm in diameter (Figure 3). Histologically, the biopsy specimens of the involved colon showed diffuse proliferation of small and medium sized atypical lymphoid cells (ALCs), suggesting malignant lymphoma (Figure 4). The tumor was featured by massive coagulative necrosis and lymphoepithelial lesions (LEL), which was characterized by the penetration of ALCs into the mucosa and destroying the mucosal glands. Moreover, there were mixed inflammatory infiltrates containing small lymphocytes, plasmacytes, neutrophils, eosinophils and histocytes in the histological background. Further immunohistochemistry confirmed these ALCs to be positive for CD56 (Figure 5A), CD3ε (Figure 5B) and granzyme B (Figure 5C), which indicated NK/T cell lymphoma. The presence of Epstein-Barr virus (EBV) infection in lymphoid cells was demonstrated by in situ hybridization for EBV-encoded early small RNA. Abdominal CT scan at readmission showed circumferential thickening of the stomas wall with enlargement of several lymph nodes around the abdominal aorta and mesentery. A bone marrow smear showed no infiltrate of abnormal cell.
During the next 2 months, the patient received a systemic chemotherapy with a combination of cyclophosphamide, doxorubicin, vincristine, and prednisone at first, and a protocol including methotrexate, ifosfamide, L-asparaginase and etoposide later. However, he died of infectious shock caused by enterotoxigenic Escherichia coli one month after the last systemic chemotherapy.
Discussion
Extranodal NK/T cell lymphoma is a subgroup of cytotoxic T or NK cell lymphoma with multiple morphologic spectrum. It is generally known that there is a preponderance of nasal type NK/T cell lymphoma in Asian, Central and South American, and the tumor usually favors young men with poor prognosis (3-6). The intestinal NK/T cell lymphoma is endoscopically featured by focally, multifocally or diffusely distributed pleomorphic irregular ulcers, which most frequently involve the ileocecum and colon (4-7). NK/T cell lymphomas developing in different sites, such as nasal cavity, nasopharynx, testis and gastrointestinal tract, share the similarities of histopathology and immunophenotype (8,9). Histologically, the tumor is characterized by infiltrates of pleomorphic ALCs, coagulative necrosis and LEL. Most commonly, the neoplasms are admixed with diverse inflammatory infiltrates. Additionally, angiocentric infiltrate may be found in some cases. Immunologically, the ALCs are positive for CD56, CD3ε and cytotoxic associated antigen such as Granzyme B, T cell intracellular antigen 1 and perforin. NK/T cell lymphoma is strongly associated with EBV infection, with infectious frequencies ranging from 76.0% to 97.6% in certain Asian populations (7,10). It has been recognized that EBV may play a “start-up and promotion” role in pathogenesis of NK/T cell lymphoma other than a second event.
The diverse clinical, endoscopic and histological presentations of primary intestinal NK/T cell lymphoma, CD and ITB may mimic each other so much that it becomes difficult to differentiate among them. In Asian Pacific region, such as China, both intestinal NK/T cell lymphoma and ITB are prevalent, and the incidence of CD has also been increased over recent decades in these areas, so differential diagnosis among them become challenging. Clinically, intestinal NK/T cell lymphoma has an aggressive course with poor outcome, while ITB can be cured by appropriate antituberculous drugs and CD has a remitting/relapsing or persistent course and usually stays lifelong. Immunosuppressive treatments with a misdiagnosis of CD may worsen ITB and may delay the diagnosis of malignant lymphoma in such patients, so we performed exploratory laparotomy just after the ruling out of ITB. Histologically, intestinal primary NK/T cell lymphoma may be confused with inflammatory or infectious disorders for several reasons. First, the florid inflammatory infiltrates of NK/T cell lymphoma may resemble the atypical histological appearance of inflammatory bowel disease (IBD) or ITB. Second, it is difficult to distinguish the predominant small or medium sized ALCs from the reactive lymphocytes in inflammatory or infectious process. Finally, the early presence of ALCs usually clustered deeply in the submucosa or much deeper, which makes it difficult to obtain such small and deep minute tumors by the forceps. Thus, close endoscopic surveillance can be of great importance in long-standing intestinal ulcerative lesions.
Whether patients with IBD are at increased risk of malignant lymphoma remains controversial. A recent study by Askling et al. (11) suggested that patients with CD carried a risk 30% higher than the general population. The exact role of chronic intestinal inflammation in the pathogenesis of these tumors is still unclear. Hall (12) and Holubar (13) reviewed the clinicopathologic features of primary malignant lymphoma complicated by IBD and indicated that lymphoma usually occurred in patients with long-standing (mean 9.1 and 6.0 years, respectively) disease. Noteworthy was the fact that lymphomas occurred in areas chronically affected by active IBD in 80% to 100% of the reported cases (12,13). This suggested a causal association between chronic inflammation and lymphoma. In addition, EBV infection may also be a risk factor for primary intestinal lymphoma, as demonstrated by Dayharsh (14), who found that IBD patients who were treated with thiopurines and subsequently developed lymphoma were more likely to have tumors positive for EBV. Moreover, the relationship between malignant lymphoma and immunosuppresive therapy in patients with CD is of increasing interest. A meta-analysis (15) of six studies showed a four-fold increase in the risk of lymphoma associated with thiopurine use, and often associated with infection of EBV. Another recent meta-analysis (16) revealed that patients with IBD who received anti-TNF agents in combination with thiopurines also had an increased risk of NHL of 6.1 per 10,000 patient-years. Such a finding needs to be compared with an expected rate of NHL of 1.9 per 10,000 patient-years in the general population and 4.0 per 10,000 patient-years in patients with CD who were treated with immunomodulators alone.
It was worth thinking about the relationship between CD and NK/T cell lymphoma in this patient. Was it the florid inflammation infiltrates that masked the deeply clustered minute lymphoma which might have coexisted with CD? Otherwise, was the NK/T cell lymphoma a complication of CD? We prefer the latter because more typical histological features of CD presented in operative specimens, and no evidence of malignant lymphoma was observed on reexamination of previous hematoxylin-eosin (H&E) and immunohistochemical pictures by experienced pathologists. Although the patient received azathioprine, it was not likely the main cause of malignant lymphoma because of the short treatment course and low dosage. The interval (7 months) between the diagnosis of CD and NK/T cell lymphoma was much shorter than that had been reported (6.0 to 9.1 years) (12,13). What triggered or drove the lymphomatous transformation in the setting of CD? And how could clinicians appreciate the turning point for early diagnosis or optimal intervention? To address these questions, revealing the nature of lymphomagenesis in relation to inflammation is of great significant.
In summary, intestinal NK/T cell lymphomas may masquerade as CD or infectious disorders. On the other hand, there may be some potential causal association between CD and malignant lymphomas, although the evolution from chronic inflammation to lymphomas remains unclear. Therefore, the initial clinically suspected malignant lymphoma with negative histological evidence should not be given up completely. Endoscopy with deep biopsies is strongly recommended in patients with a change in clinical condition and as part of the evaluation of medically refractory disease. Further immunophenotypic studies and T cell receptor gene rearrangement should be investigated if any atypical lymphatic proliferation exists, and if necessary, exploratory laparotomy should be determined in time.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Nakamura S, Matsumoto T, Iida M, et al. Primary gastrointestinal lymphoma in Japan: a clinicopathologic analysis of 455 patients with special reference to its time trends. Cancer 2003;97:2462-73.
- Kohno S, Ohshima K, Yoneda S, et al. Clinicopathological analysis of 143 primary malignant lymphomas in the small and large intestines based on the new WHO classification. Histopathology 2003;43:135-43.
- Suzuki R. Leukemia and lymphoma of nature killer cells. J Clin Exp Hematopathol 2005;45:51-70.
- Ko YH, Cho EY, Kim JE, et al. NK and NK-like T-cell lymphoma in extranasal sites: a comparative clinicopathological study according to site and EBV status. Histopathology 2004;44:480-9.
- Kim HS, Lee DK, Baik SK, et al. Primary CD56+ T/NK cell lymphoma of the colon. J Gastroenterol 2002;37:939-46.
- Tung CL, Hsieh PP, Chang JH, et al. Intestinal T-cell and natural killer-cell lymphomas in Taiwan with special emphasis on 2 distinct cellular types: natural killer-like cytotoxic T cell and true natural killer cell. Hum Pathol 2008;39:1018-25.
- Zhang WY, Li GD, Liu WP, et al. Features of intestinal T-cell lymphomas in Chinese population without evidence of celiac disease and their close association with Epstein-Barr virus infection. Chin Med J (Engl) 2005;118:1542-8.
- Schwartz EJ, Molina-Kirsch H, Zhao S, et al. Immunohistochemical characterization of nasal-type extranodal NK/T-cell lymphoma using a tissue microarray: an analysis of 84 cases. Am J Clin Pathol 2008;130:343-51.
- Lin X, Li D, Xie P, et al. Primary testicular NK/T-Cell lymphoma: a study of two cases and review of literature. Chin J Cancer Res 2010;22:239-44.
- Ng SB, Lai KW, Murugaya S, et al. Nasal-type extranodal natural killer/T-cell lymphomas: a clinicopathologic and genotypic study of 42 cases in Singapore. Mod Pathol 2004;17:1097-107.
- Askling J, Brandt L, Lapidus A, et al. Risk of haematopoietic cancer in patients with inflammatory bowel disease. Gut 2005;54:617-22.
- Hall CH Jr, Shamma M. Primary intestinal lymphoma complicating Crohn’s disease. J Clin Gastroenterol 2003;36:332-6.
- Holubar SD, Dozois EJ, Loftus EV Jr, et al. Primary intestinal lymphoma in patients with inflammatory bowel disease: a descriptive series from the prebiologic therapy era. Inflamm Bowel Dis 2011;17:1557-63.
- Dayharsh GA, Loftus EV Jr, Sandborn WJ, et al. Epstein-Barr virus-positive lymphoma in patients with inflammatory bowel disease treated with azathioprine or 6-mercaptopurine. Gastroenterology 2002;122:72-7.
- Kandiel A, Fraser AG, Korelitz BI, et al. Increased risk of lymphoma among inflammatory bowel disease patients treated with azathioprine and 6-mercaptopurine. Gut 2005;54:1121-5.
- Siegel CA, Marden SM, Persing SM, et al. Risk of lymphoma associated with combination anti-tumor necrosis factor and immunomodulator therapy for the treatment of Crohn’s disease: a meta-analysis. Clin Gastroenterol Hepatol 2009;7:874-81.