Efficacy and safety evaluation of icotinib in patients with advanced non-small cell lung cancer
Introduction
Lung cancer is among the most commonly diagnosed cancers worldwide, representing the first cause of cancer-related death (1). Non-small cell lung cancer (NSCLC) accounts for approximately 80% of all lung cancers, being often diagnosed at an advanced stage when treatment options are limited (2). First-line chemotherapy for NSCLC patients with advanced disease is generally platinum-based, yielding a median overall survival of 8-11 months (3).
Since its identification in 1986, the epidermal growth factor receptor (EGFR) has emerged as a crucial factor for the development and growth of human malignancies, including lung cancer (4). In fact, EGFR signal transduction network plays an important role in multiple tumorigenic processes such as proliferation of cancer cells, angiogenesis, and metastasization (5). The discovery of activating EGFR mutations in NSCLC has led to a shift in treatment paradigm for some patients with advanced disease (6). Mutations in exons 18-21 in the tyrosine kinase domain are associated with improved clinical outcomes following treatment with tyrosine kinase inhibitors (TKIs) (7).
EGFR belongs to the Erb family of transmembrane receptor tyrosine kinases which includes also HER2 (ErbB2), HER3 (ErbB3) and HER4 (ErbB4). Upon ligand binding, EGFR undergoes homo- or hetero-dimerization with other receptors of the same family with subsequent autophosphorylation and activation of the intracellular tyrosine-kinase (TK) domain, recruitment of second messengers and intensification of the anti-apoptotic signaling (8). Interestingly, no ligand has been identified for the HER2 orphan receptor while no kinase activity has been documented for HER3, which allow both HER2 and HER3 to be actively involved in EGFR-mediated signaling as preferred hetero-dimerization partners of EGFR itself (9). There are several ways through which EGFR can be aberrantly activated including receptor overexpression, gene amplification and gene mutation (10). However, because of its crucial role as oncogenic determinant, the presence of an activating (meaning ligand-independent activation of the TK) EGFR mutation in NSCLC carries major therapeutic implications (11). Icotinib hydrochloride is a selective epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI). Previous studies have shown that EGFR-TKI treatment is safe and efficacious in patients with advanced NSCLC for whom platinum-based chemotherapy had failed, and EGFR-TKI treatment results in an improvement in patients’ quality of life and extension of survival (12-14). From August 2011 to June 2012, 89 patients with advanced NSCLC were enrolled in this retrospective study, and all of them were treated with icotinib hydrochloride. The purpose of this study was to detect the efficacy and safety of the treatment with icotinib in patients with advanced NSCLC.
Patients and methods
Patient characteristics
Of the 89 patients with stage IIIB or IV NSCLC, 49 were male and 40 were female. The age of the patients ranged from 40 to 85 years, with a median age of 63 years. All cases were validated as NSCLC using cytological or histological methods including 75 cases of adenocarcinoma, 12 cases of squamous carcinoma, and 2 cases of poorly differentiated carcinoma. According to the Union for International Cancer Control lung cancer staging system (1997 version), 3 patients were at stage IIIB and 86 patients were at stage IV. Fifty-two patients were smokers and 37 were non-smokers. Forty patients had been given 1 chemotherapy regimen, 32 had been given 2 or more, and 17 had not been given any chemotherapy. The 17 previously untreated patients were of advanced age and were either reluctant about or intolerant of chemotherapy. Six of the 89 patients had their tumor tissue tested for the EGFR mutations, of which 3 had a deletion mutation in exon 19, 1 had a point mutation in exon 21, and 2 had a wild-type EGFR gene (Table 1). All patients had at least 1 measurable lesion. During the administration of icotinib, we performed blood, liver, and renal function tests for the patients at regular intervals. Regular imaging of the tumor lesion was required in addition to follow-up examination.
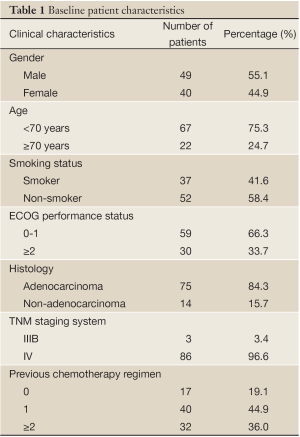
Full table
Study treatment
Icotinib hydrochloride (Zhejiang Beta pharmaceutical Co., Ltd., Hangzhou, China) was administered at a dose of 125 mg 3 times a day until disease progression or presentation of unacceptable toxicity. No other systematic anticancer treatment was administered concurrently with icotinib treatment.
Response to treatment and adverse effects
Therapeutic evaluations were performed according to the Response Evaluation Criteria in Solid Tumors (RECIST) and included complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). The response rate (RR) comprises CR and PR. The disease control rate (DCR) comprises CR, PR, and SD. The toxic effects were divided into 5 grades ranging from 0 to 4 based on the World Health Organization (WHO) toxicity criteria (1981 version). The first objective therapeutic evaluation was performed 4 weeks after drug administration. Thereafter, evaluations were performed every 8 weeks or at the appearance of a new symptom or the progression of an original symptom.
Statistical analyses
The chi-square (χ2) test was used for intergroup comparisons of RR and DCR at a significance level of 5% (α=0.05). The statistical data were obtained using an SPSS software package (SPSS 11.5 Inc., Chicago, IL, USA).
Results
Efficacy
One (1.1%) patient achieved CR, 31 (34.8%) patients achieved PR, 30 (33.7%) patients achieved SD, and 27 (30.3%) patients exhibited PD. The RR was 36.0% (32/89), and the DCR was 69.7% (62/89). The RR and DCR were significantly higher in adenocarcinoma patients than in the non-adenocarcinoma patients (P<0.05). Of the 4 patients with EGFR mutations, 2 achieved PR, 1 achieved SD, and 1 exhibited PD. The RR of patients with EGFR mutations was 50.0% (2/4) and the DCR of these patients was 75.0% (3/4). The data are provided in Table 2.
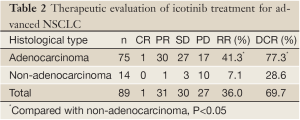
Full table
Symptom improvement
Fifty-one (57.3%) of 89 patients exhibited symptomatic improvement, specifically for symptoms of cough, pain, chest distress, and dyspnea, and an improvement in Eastern Cooperative Oncology Group (ECOG) performance status was noted. The median time from first drug administration to symptomatic improvement was 2 weeks.
Toxicity
The main toxicities were rash [30 (33.7%) of 89 patients] and diarrhea [15 (16.9%)] of grade I-II. Rash mainly appeared on the face and thoracic (dorsal) region. Most rashes were red papules and were self-healing. Diarrhea was generally relieved after symptomatic treatment. Dry skin was noted in 6 (6.7%) patients, and oral ulcers were observed in 5 (5.6%) patients. Five (5.6%) patients exhibited liver dysfunction, which recovered after 1 week of drug discontinuation and liver supportive therapy. Icotinib was continued after liver function recovery. Fatigue was noted in 3 (3.4%) patients, and paronychia, in 1 (1.1%) patient. There were no cases of interstitial pneumonia and no drug-related deaths. None of the patients discontinued icotinib because of intolerance to toxic effects. The data are shown in Table 3.
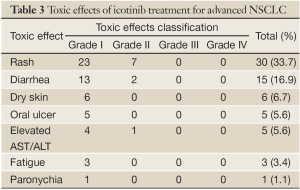
Full table
Discussion
The EGFR is a transmembrane tyrosine kinase receptor that is often found in epidermal cell tumors. The signaling pathway of EGFR is closely related with several pivotal drivers of malignancy including tumor cell proliferation, apoptosis, angiogenesis, invasion, and metastasis (15). Because EGFR is often overexpressed in NSCLC (16,17), small molecular EGFR-TKIs represent new therapeutic options for advanced NSCLC. The EGFR-TKIs gefitinib and erlotinib specifically target and inhibit EGFR-TK activity and are non-cytotoxic. EGFR-TKIs regulate tumor cell growth, metastasis, and angiogenesis, and they promote tumor cell apoptosis (12,13). In several large-scale clinical trials, EGFR-TKIs presented excellent efficacy and safety in NSCLC patients who were insensitive to previous chemotherapies. Currently, EGFR-TKIs are recommended worldwide as a standard second-line regimen for advanced NSCLC (13,18,19).
Icotinib hydrochloride is a selective EGFR-TKI for oral usage. Recent research indicated that icotinib hydrochloride has excellent anticancer efficacy and safety (14,20-22). A phase III trial (ICOGEN) was performed using a randomized, double-blind, multicenter, controlled, head-to-head (icotinib vs. gefitinib) study design (14). ICOGEN defined gefitinib-treated patients as positive controls and enrolled 399 patients with advanced NSCLC that exhibited tumor relapse or metastasis (stage IIIB or IV). Of these patients, 200 were treated with icotinib and 199 were treated with gefitinib. The results indicated that the efficacy differences were not significant between the icotinib-treated group and the gefitinib-treated group. The objective RR (ORR) of the icotinib group was 27.6% versus 27.2% in the gefitinib group. The DCR of the icotinib group was 75.4% versus 74.9% in the gefitinib group. Thus, the ORR and DCR were quite similar between the 2 groups. The PFS in the icotinib group was 4.6 months versus 3.4 months in the gefitinib group. With regard to safety, icotinib hydrochloride was superior to gefitinib. ICOGEN demonstrated the safety and efficacy of icotinib for advanced NSCLC patients for whom platinum-based chemotherapy had failed, which improved the patients’ quality of life and prolonged their survival.
Our study demonstrated that icotinib hydrochloride benefited all types of advanced NSCLC, with the best efficacy observed in adenocarcinoma patients. In the 89 assessable patients, the RR and DCR were 36.0% and 69.7%, respectively, and were significantly higher in adenocarcinoma patients than in non-adenocarcinoma patients (P<0.05). Research has shown that EGFR mutations are critical for EGFR-TKI sensitivity in lung cancer patients. Additionally, EGFR mutations are predictive of the response of patients with advanced NSCLC to erlotinib or gefitinib treatment (23-25). In the 4 cases of EGFR mutations examined in our study, the RR and DCR were 50.0% and 75.0%, respectively. One patient with a point mutation in exon 21 presented PD. Of the 2 patients with wild-type EGFR, one presented SD and the other presented PD. On the basis of the above data, we concluded that the icotinib regimen would be considerably advantageous for patients with the EGFR mutation, and patients with the wild-type EGFR gene could also benefit to some extent. In addition to its effects on the targeted lesion, icotinib hydrochloride can significantly improve systematic symptoms including cough, pain, chest distress, dyspnea, and ECOG performance status. Hence, the patients’ quality of life was improved.
In our study, we found that icotinib hydrochloride was moderately effective for the treatment of brain metastasis of lung cancer. Four out of 14 patients exhibiting brain metastasis who received icotinib treatment after whole brain radiation therapy achieved PR. Five of the remaining patients exhibited SD. The significant symptom relief observed in brain metastasis patients indicated that icotinib can cross the blood brain barrier and be used in NSCLC patients with brain metastasis. However, in 2 cases of SD, brain metastasis occurred while the lung lesion remained stable during icotinib treatment. This contradictory data implies that brain metastasis was not related to drug resistance. Additional research will be required to explain this phenomenon. The main toxic effects observed during our study included rash and diarrhea, at morbidities of 33.7% and 16.9%, respectively. Most of the toxic effects were of grade I or II and were reversible. Five patients exhibited transient liver dysfunction that did not require drug discontinuation. In our study, icotinib showed limited toxicity and excellent tolerability and did not damage major organs; these results cannot be achieved with traditional chemotherapy.
In conclusion, icotinib hydrochloride showed definite efficacy in the treatment of advanced NSCLC with little toxicity. Icotinib was well tolerated, effectively relieved symptoms, and improved life quality. As an EGFR-targeted agent, icotinib hydrochloride represents a safe and efficacious new therapeutic option for patients with tumor relapse or metastasis, patients who are intolerant of or reluctant about chemotherapy, and adenocarcinoma patients in particular.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90.
- Méndez M, Custodio A, Provencio M. New molecular targeted therapies for advanced non-small-cell lung cancer. J Thorac Dis 2011;3:30-56.
- Ramalingam S, Belani C. Systemic chemotherapy for advanced non-small cell lung cancer: recent advances and future directions. Oncologist 2008;13:5-13.
- Huang SM, Harari PM. Epidermal growth factor receptor inhibition in cancer therapy: biology, rationale and preliminary clinical results. Invest New Drugs 1999;17:259-69.
- Metro G, Finocchiaro G, Toschi L, et al. Epidermal growth factor receptor (EGFR) targeted therapies in non-small cell lung cancer (NSCLC). Rev Recent Clin Trials 2006;1:1-13.
- Enting D, Spicer J. EGFR inhibition and more: a new generation growing up. J Thorac Dis 2012;4:553-5.
- Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A 2004;101:13306-11.
- Mendelsohn J, Baselga J. Epidermal growth factor receptor targeting in cancer. Semin Oncol 2006;33:369-85.
- Metro G, Crinò L. Advances on EGFR mutation for lung cancer. Transl Lung Cancer Res 2012;1:5-13.
- Chou T, Finn RS, Garon EB. Expanding options for EGFR targeting in lung cancer. Transl Lung Cancer Res 2012;1:287-8.
- Pennell NA. Treating acquired resistance to EGFR-tyrosine kinase inhibitors: Still a work in progress. Transl Lung Cancer Res 2012;1:149-51.
- Han SW, Hwang PG, Chung DH, et al. Epidermal growth factor receptor (EGFR) downstream molecules as response predictive markers for gefitinib (Iressa, ZD1839) in chemotherapy-resistant non-small cell lung cancer. Int J Cancer 2005;113:109-15.
- Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005;353:123-32.
- Sun Y, Shi Y, Zhang L, et al. A randomized, double-blind phase III study of icotinib versus gefitinib in patients with advanced non-small cell lung cancer (NSCLC) previously treated with chemotherapy (ICOGEN). J Clin Oncol 2011;29:abstr 7522.
- Normanno N, Bianco C, De Luca A, et al. Target-based agents against ErbB receptors and their ligands: a novel approach to cancer treatment. Endocr Relat Cancer 2003;10:1-21.
- Salomon DS, Brandt R, Ciardiello F, et al. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol 1995;19:183-232.
- Fontanini G, De Laurentiis M, Vignati S, et al. Evaluation of epidermal growth factor-related growth factors and receptors and of neoangiogenesis in completely resected stage I-IIIA non-small-cell lung cancer: amphiregulin and microvessel count are independent prognostic indicators of survival. Clin Cancer Res 1998;4:241-9.
- Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet 2008;372:1809-18.
- Tassinari D, Carloni F, Santelmo C,et al. Second line treatments in advanced platinum-resistant non small cell lung cancer. A critical review of literature. Rev Recent Clin Trials 2009;4:27-33.
- Tan F, Shen X, Wang D, et al. Icotinib (BPI-2009H), a novel EGFR tyrosine kinase inhibitor, displays potent efficacy in preclinical studies. Lung Cancer 2012;76:177-82.
- Tan FL, Zhang L, Zhao Q, et al. Pharmacology and clinical evaluation of Icotinib Hydrochloride. Chinese Journal of New Drugs 2009;18:1691-700.
- Zhao Q, Shentu J, Xu N, et al. Phase I study of icotinib hydrochloride (BPI-2009H), an oral EGFR tyrosine kinase inhibitor, in patients with advanced NSCLC and other solid tumors. Lung Cancer 2011;73:195-202.
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57.
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8.
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42.


