Role of contrast-enhanced ultrasonography in percutaneous radiofrequency ablation of liver metastases and efficacy evaluation
Introduction
Percutaneous radiofrequency ablation (RFA) is one of the most widely used minimally invasive local treatment developed in recent years, which has been recognized as a safe and efficient therapeutic method for primary and secondary hepatic malignant tumors (1-5). Ultrasonography (US) guidance is performed in real time without radiation and is recognized as the most convenient imaging modality for percutaneous RFA treatment in patients with liver malignancies. However, not all tumors that are detected by computed tomography (CT) or magnetic resonance imaging (MRI) can be clearly demonstrated by unenhanced US (6,7).
Contrast-enhanced ultrasonography (CEUS) was initially used to detect and differentiate liver focal lesions (8,9). It had been reported that CEUS significantly improved the detection of liver metastases compared to unenhanced US (10,11). In 1999, Solbiati, et al. (12) firstly reported a clinical application about using the ultrasonography contrast agent Levovist 24 h after RFA treatment with liver metastases to early detect residual tumor. After that, a few of studies have shown inspiring results for CEUS used in RFA treatment in patients with liver malignancies, including detecting and locating obscure lesions on the unenhanced US before RFA (13,14), guiding and monitoring the treatment process (15), and evaluating the early outcome of RFA (12,16). We had also reported the role of CEUS in planning treatment protocols and selection patients with hepatocellular carcinoma (HCC) for percutaneous RFA therapy (17,18). These studies offered valuable information for us. In this study, CEUS was used for patients with liver metastases especially patients after chemotherapy in order to qualify the tumor number, location, and infiltration range.
Based on the former studies, we performed a retrospective analysis from prospective database of the ultrasound department of Beijing Cancer Hospital with a study purpose to analyze the application value of CEUS for percutaneous RFA of liver metastases in terms of metastatic tumor detection and tumor size. The long-term outcome of RFA using CEUS was evaluated at the meantime, which was compared with the historical control group without using CEUS. Furthermore, the two groups were subdivided according to tumor size (≤2 cm or >2 cm) and number (solitary or multiple) to specifically analyze the effect of tumor size and number on tumor recurrence and survival between two groups.
Materials and methods
Patients
Our study was approved by the ethics committee of Beijing Cancer Hospital. Written informed consent was obtained from all patients prior to the CEUS and RFA procedures. One hundred and thirty-six consecutive patients with 219 liver metastases who received CEUS examination 1 h before RFA at the ultrasound department between May 2004 and September 2010 served as a CEUS group. To assess whether CEUS facilitated RFA, a historical control group was set to compare the therapeutic efficacy between two groups. One hundred and twenty-six consecutive patients with 216 liver metastases in the control group were treated by percutaneous RFA with unenhanced US between July 2000 and April 2004, which was prior to the introduction of CEUS. Written informed consent was also obtained from patients in the control group before RFA. For two groups, liver metastasis was diagnosed based on the clinical history, enhanced CT or MRI, and/or histopathologic findings by biopsy. RFA was selected when patients were not candidates for surgery because of the patients’ comorbidity, tumor location or patients preferred to receive RFA treatment. Patients with liver metastases were recruited to the study if they met the following criteria: (I) a solitary liver metastasis of less than 7 cm, (II) ≤5 tumors of ≤5 cm in maximum diameter, (III) platelet count of greater than 50,000/µL and prothrombin time of greater than 50%, (IV) Child-Pugh classification A or B, or (V) the primary tumor and/or extrahepatic metastases of all patients had been resected or sustained stable after treatment. End-point of the study was December 2010. The mean age of 136 patients in the CEUS group was (59.1±10.9) years (range, 32-90 years), and the mean age of 126 patients in the control group was (59.2±11.0) years (range, 30-81 years) (P=0.950). The mean tumor size was (3.2±1.2) cm (range, 0.7-6.7 cm) and (3.4±1.0) cm (range, 1.0-6.7 cm) for the CEUS group and the control group, respectively (P=0.084). The tumor number ranged from 1-5, with (1.6±0.8) tumors per patient of the CEUS group and (1.7±1.1) tumors of the control group, respectively (P=0.595). The primary sites included colon-rectum, breast, stomach, lung, esophagus, pancreas and so on. Most liver metastases originated from colon-rectum, 44.1% (60/136 patients) in the CEUS group and 51.6% (65/126 patients) in the control group, respectively (P=0.547). No statistically significant differences were found with respect to other baseline clinical characteristics between two groups (Table 1).
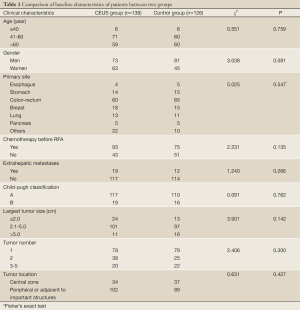
Full Table
Examination method and equipment
Ultrasonography systems included a LOGIQ 9 (GE Medical Systems, Milwaukee, WI, USA) with a 2.5-5.0-MHz convex-arrayed transducer, and an Aloka α-10 (Aloka Co., Ltd, Tokyo, Japan) with a 2.5-5.0-MHz small-sector and board-view convex probe. All liver metastases in two groups were first recognized and recorded with tumor location, size, number and color flow by unenhanced US. In the CEUS group, the contrast agent SonoVue (Bracco SpA, Milan, Italy) was used (19). A homogeneous microbubble suspension was formed with 5 mL of saline. The contrast media was manually administered intravenously through a 20 gauge cannula into the antecubital vein at a 2.4 mL bolus within 1-3 s and then flushed with 10 mL of normal saline. The acoustic power output was set at the default setting with a mechanical index of 0.09. A single focus point was set at the deepest point of the monitor.
After injection of the contrast media, the target tumor was firstly observed and then the whole liver was scanned in the liver parenchyma phase to find any new hypoechoic liver metastases. The whole time of CEUS procedure was 6-8 min. If any lesion was suspected, 2.4 mL was administered once more. The process of CEUS was observed real-timely and recorded by digital imaging and communication in medicine (DICOM). The tumor location, size, number and relationship with the surrounding structures (such as the gastrointestine, diaphragm, large vessels, etc.) were written down in detail by review. The CEUS examinations were performed by experienced radiologists having had more than 100 cases of liver CEUS examinations.
RFA treatment procedure
The Aloka α-10 ultrasonography system with 2.5-5.0-MHz small-sector and board-view convex probe equipped with attachments for biopsy and RFA electrode insertion was used to guide the RFA therapy.
Two types of RFA systems were used according to the tumor size, shape and location. One was a 460-KHz of maximum power 200 W generator (Model 1500; RITA Medical System, Mountain View, CA, USA) with the expandable electrodes, which deployed to form a spherical ablation ranged from 2.0 to 5.0 cm in diameter. The other type of RF ablation system was a (470±10)-KHz output frequency of 250 W maximum power generator regulated by power (CelonLab POWER RF ablation system, Germany). The 15-20 cm electrode needles with a 3 or 4 cm exposed tip were connected to the system in bipolar and multipolar mode. The scope and shape of the ablation zone were depended on the length of the needle tip, number of needles, distance between the needles, the emission power and the treatment time.
The RFA was performed percutaneously by two radiologists with more than 5 years experience in US-guided interventional procedures including RFA. All patients of two groups underwent contrast-enhanced CT or MRI scan within 1 month before RFA. In the CEUS group, the RFA protocol for each case was designed according to CEUS findings combined with enhanced CT or MRI, while in the control group, the RFA protocol was designed according to unenhanced US and contrast CT/MRI results. For all patients, moderate sedation anesthesia (2.5-5.0 mg midazolam; 50-100 µg fentanyl) and local anesthesia (5-15 mL 1% lidocaine) were used during RFA procedure. The patients’ vital signs were continuously monitored. The electrode needle was inserted along a predetermined puncture line into the target tumor. During the whole process, the ablated area was monitored with unenhanced US. The tumor was considered to be completely ablated if the zone of increased echogenicity beyond the scope of the tumor size at US. It was necessary for overlapping ablations when the tumor was larger so that the whole tumor and a margin of at least 0.5-1.0 cm diameter around the tumor were ablated (20). Track ablation was performed when withdrawing the electrode in all patients. These patients were observed 30 min after RFA until there was no evidence of active bleeding on the US scan. Generally, the patients were hospitalized for 1-3 d after RFA.
Efficacy evaluation and follow-up
An enhanced CT or MRI scan together with laboratory tests was performed 1 month after the first RFA treatment to evaluate the tumor necrosis. Imaging and laboratory examination follow-ups were conducted once every 2-3 months within the first year, and then once every 4-6 months in the following years. Early tumor necrosis was considered if no enhancement was seen within or around the ablated tumor on one month CT/MRI. A nodular enhancement in the liver was considered recurrence, which was classified as local recurrence (LR, within or at the periphery of the ablated site) and distant intrahepatic recurrence (DIR, remote from the RFA site). CT examinations were performed with a GE LightSpeed 64-slice spiral CT scanner. Magnetic resonance imaging examinations were performed with a GE EchoSpeed 1.5 T MRI scanner. Two radiologists who had at least 5 years experience in liver CT or MRI reviewed the results to assess RFA efficacy with consensus.
Statistical analysis
Data were expressed as 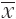 ±s. The Chi-square, Fisher’s exact and unpaired Student’s t tests were used for the two groups in contrast to the clinical characteristics and recurrence results. We used the Kaplan-Meier method and the log-rank test to calculate and compare survival outcomes, including overall survival (OS) and local recurrence-free survival (LRFS). Survival time was measured in months from the point of liver metastases initially treated by RFA. All P values were derived from two-tailed tests, and a level of less than 0.05 was accepted as statistically significant. SPSS statistical analysis software version 13.0 (SPSS, Inc., Chicago, IL, USA) was used.
±s. The Chi-square, Fisher’s exact and unpaired Student’s t tests were used for the two groups in contrast to the clinical characteristics and recurrence results. We used the Kaplan-Meier method and the log-rank test to calculate and compare survival outcomes, including overall survival (OS) and local recurrence-free survival (LRFS). Survival time was measured in months from the point of liver metastases initially treated by RFA. All P values were derived from two-tailed tests, and a level of less than 0.05 was accepted as statistically significant. SPSS statistical analysis software version 13.0 (SPSS, Inc., Chicago, IL, USA) was used.
Results
Comparison of tumor size and number in CEUS group
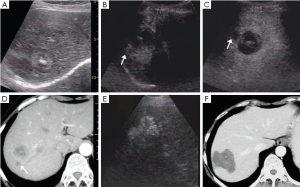
The largest tumor in 136 patients of the CEUS group was set for the target tumor. The maximum diameter of the target tumor measured in the same section was compared on unenhanced US and on CEUS. Two of 136 tumors were not visualized on unenhanced US. Sixty-three (47.0%) of the remaining 134 tumors examined with CEUS were 0.3 cm larger than with unenhanced US (range, 0.4-2.0 cm; 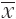 ±s, 0.9±0.5 cm). Among them, 90.5% (57/63) were larger than 2 cm (Figure 1).
±s, 0.9±0.5 cm). Among them, 90.5% (57/63) were larger than 2 cm (Figure 1).
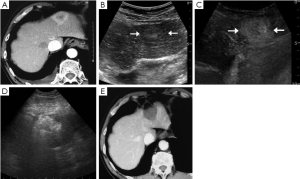
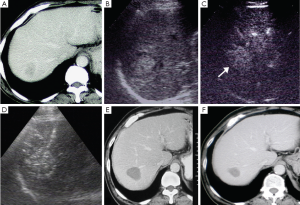
Besides, additional 40 metastatic lesions (range, 1-3 lesions per patient) were detected in 25 patients on CEUS, with (1.8±0.6) cm (range, 0.7-3.0 cm) in diameter, and 82.5% (33 of 40 lesions) occurred in lesions ≤2.0 cm (Figure 2).
Liver metastases necrosis and recurrence
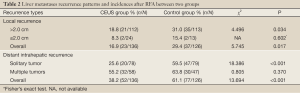
The follow-up period was 3.0-79.0 (16.3±13.8) months for the CEUS group and 3.0-97.0 (21.9±18.0) months for the control group. The early tumor necrosis rate after the first RFA treatment was higher in the CEUS group with 215 (98.2%) of 219 lesions than in the control group with 204 (94.4%) of 216 lesions (P=0.039). The differences in recurrence results between two groups are demonstrated on Table 2. LR and DIR both less often occurred in the CEUS group compared with the control group (P=0.017 and P<0.0001).
Full Table
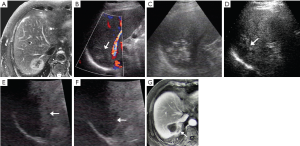
In a separate analysis of patients with tumors ≤2.0 cm, there was no significant difference of incidence of LR between CEUS group (8.3% vs. 15.4%, P=0.602). But for patients with tumors >2.0 cm, LR was more common in the control group (31.0% vs. 18.8%, P=0.034) (Figure 3). Besides, the relationship between the incidence of DIR and the liver metastatic tumor number with initial treatment in two groups were analyzed. For the solitary liver metastasis, the CEUS group showed a significantly lower DIR rate of 20 (25.6%) of 78 patients than the control group of 47 (59.5%) of 79 patients (P<0.001). There was no statistically difference of DIR rate between two groups in terms of multiple liver metastases (P=0.370).
The recurrence time was (6.5±5.8) months (range, 2.0-25.0 months) for the CEUS group and (5.2±4.1) months (range, 2.0-13.0 months) for the control group, respectively (P=0.100).
Survival analysis
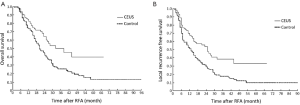
At the end of the study, 117 (44.7%) patients survived, 123 (46.9%) died, and 22 (8.4%) patients dropout among 262 patients. The OS rates for two groups are presented in Figure 4A. The 1-, 2- and 3-year OS rates were 82.5%, 64.3% and 50.1% respectively for the CEUS group, and 73.5%, 44.9% and 25.3% respectively for the control group. The median survival time was 38 and 20 months for the CEUS and control groups, respectively (P=0.002). In the analysis of LRFS, the CEUS group showed significantly higher 1-, 2- and 3-year LRFS rates (67.7%, 53.8% and 38.3%, respectively) than control group (51.2%, 31.1% and 19.3%, respectively) (P<0.001, Figure 4B).
The survival rate and LFRS rate of the patients with tumors larger than 2.0 cm were separately assessed, which were both higher in the CEUS group. The 3-year survival rate and the 3-year LFRS rate were 40.6% and 34.7% for the CEUS group (n=113), respectively, compared with 26.9% and 18.6% for the control group (n=112), respectively (P=0.026 and 0.007) (Figure 5A,B). As for tumors ≤2.0 cm, the patients in the CEUS group survived longer (P=0.035), whereas there was no significant difference in LFRS between two groups (P=0.050).

The survival of solitary and multiple liver metastatic tumors after the first RFA treatment between two groups was analyzed. There was no statistical difference in survival rate between two groups with multiple liver metastases (P=0.524; Figure 6A). While the CEUS group (n=78) showed a longer survival than the control group (n=79) for solitary liver metastasis (P=0.001; Figures 6B and 7).

Complications
No side-effects correlating with the contrast media of SonoVue had happened in the 136 patients of CEUS group. No therapy-related deaths were found in 262 patients. Severe complications of 2 (2/136; 1.5%) in the CEUS group and 3 (3/126; 2.4%) in the control group were observed. In the CEUS group, subcapsular hemorrhage in one patient was found during the procedure with the result of stabbing subcapsular arterial-venous shunt by electrode needles, which was confirmed by CEUS and the hemorrhage was stopped by immediately RFA at the bleeding site. The other patient suffering from pancreatic cancer had high fever up to 40.2 °C after treatment. The ablated tumor near the right diaphragm was demonstrated with infection by US and CT scan. The symptoms got improved after puncture drainage in the infective area. In the control group, there were two cases of biliary leakage which was alleviated by conservative treatment and one case of needle track seeding 12 months after RFA. There was no significant difference of incidence of complications between two groups (P=0.674).
Discussion
Imaging technique is of paramount importance in all steps of tumor ablation (15). An excellent imaging modality is crucial to achieve optimal therapeutic outcomes. Studies showed that using CEUS with SonoVue as the routine pretreatment imaging modality, there was increased conspicuity for tiny hypovascular metastases in 12% of 53 patients than enhanced helical CT which resulted in a significant modification of treatment strategy (15). In the recent literature (21), perfluorocarbon microbubbles (Sonazoid) was used for guidance RFA therapy in patients with HCC and liver metastases not clearly demarcated by unenhanced US, complete tumor necrosis was achieved by a single session of RFA in 94% patients. In the current study, the early tumor necrosis rate was 98.2% of 219 tumors in the CEUS group, which was similar to the report mentioned above. The issues about intrahepatic recurrence, patient survival and the factors of tumor size and number of two groups were discussed in detail below.
The recurrence rate of liver metastases after percutaneous RFA reached as high as 11-42% (22,23). The tumor size and number had been proposed as one of the most important factors related to the complete ablation and tumor recurrence after RFA (2). The risk of LR after percutaneous RFA is significantly higher when metastatic tumors are larger than 3.0 cm in diameter (24,25). Tumor size may be underestimated on unenhanced US for larger and poor-defined tumors (17). It easily resulted in insufficient ablation and early LR. One case in earlier period occurred LR three months after percutaneous RFA, which increased technical difficulties for retreatment and hard to achieve desired effect (Figure 3). It also had been confirmed that in resected colorectal metastases, microscopic bile duct, portal or hepatic vein invasion or peritumoural micrometastases were found in 31.0-50.0%, 9-21 mm from the macroscopic tumor edge (26-29). Our current study showed that 47.0% (63/134) of tumors examined with CEUS increased more than 0.3 cm in size compared with unenhanced US and 90.5% among them occurred in tumors larger than 2 cm. Therapeutic strategies were set according to findings on CEUS in the CEUS group and expand ablation including 0.5-1.0 cm surrounding the tumor to obtain enough safe margin was performed for two groups. Results in our study showed that the CEUS group had higher early tumor necrosis rate and lower LR rate. For tumors larger than 2.0 cm, the LR rate is lower in the CEUS compared with the control group. It was considered that CEUS can be more accurate to identify the actual tumor size and more sensitive to depict invasion range, especially very useful for larger tumors treatment (Figure 1).
In addition, confirmation of the tumor number before treatment is also critical for making a reasonable treatment plan and judging the prognosis. DIR appeared shortly after surgical resection or RFA therapy, probably because the tumors had already existed before treatment but failed to be discovered for the inspection method limitation. On the other hand, part of patients with liver metastases after chemotherapy often showed heterogeneously hepatic texture or fatty liver background, which would influence to detect metastatic tumors, especially for small foci. In terms of liver metastases, the detection sensitivity on unenhanced US varied 53-77% (11,30,31), lower to 20% for tumors less than 1.0 cm (30). In a study comparing unenhanced US in the detection of liver metastases, the average number of confirmed metastases increased from 3.06 to 5.42, the sensitivity for detecting liver metastases improved from 63% to 91% on CEUS and more importantly sub-centimeter lesions were significantly increased from 54% to 92% of confirmed cases on CEUS (32). Early study of ourselves had the similar result that CEUS was even better than enhanced CT for detection of small and minute liver metastatic lesions (33). The sensitivity was also higher to detect liver metastases for patients after chemotherapy on CEUS than on unenhanced US with 82.0% and 60.3%, respectively (34). Our study found a total of 40 additional lesions in 25 of 136 patients on CEUS, and the smallest one was 0.7 cm in diameter. All of the additional lesions were ablated together with the target tumor in the CEUS group. Follow-up results after RFA showed the DIR rates of overall and solitary liver metastases were both lower in the CEUS group than in the control group (P<0.001). It was considered that more existed or potential metastases were detected by CEUS before treatment so that we could make optimized RFA therapeutic strategies for patients, and the small lesions could be ablated at the same time (Figure 2).
The median OS was 27 months, and for those with fewer than 4 tumors smaller than 5.0 cm, the median survival was 33 months (2). Previous reports of survival following percutaneous RFA of colorectal liver metastases varied based on the patients’ selection. Solbiati, et al. (35) reported a median survival duration of 36 months with 3-year survival rate of 46% in 117 colorectal liver metastases patients with 4 or less tumors (mean, 1.5 tumors) of 0.7-9.6 cm (mean, 3.2 cm) in diameter. Gillams and Lees (2) reported a median survival of 36 months with 3-year survival of 49% and 5-year survival of 24% from ablation in 123 patients with 5 or less tumors of 5.0 cm or less maximum diameter. Meloni, et al. (3) reported survival of 52 patients with breast cancer liver metastases after percutaneous RFA, including 5 or less tumors (mean 1.7 tumors) of 5.0 cm or less largest diameter (mean, 2.5 cm), with a result of a median survival time of 29.9 months and 3, 5-year survival rates of 43.0%, 27.0%, respectively. In the current study, the median survival duration of 38.0 months and the 3-year OS of 50.1% were slightly higher than those mentioned above and significantly higher than the control group of 20.0 months and 25.3%, respectively. The 3-year LRFS rate of 38.3% for the CEUS group was also higher than 19.3% for the control group. Although there were studies (35) showed that no significant correlations were found between survival and liver metastatic tumor number and size with percutaneous RFA treatment, our results demonstrated that patients with tumor larger than 2.0 cm or with single tumor in the CEUS group survived longer than those in the control group (P=0.026 and 0.001). However, there was no significant difference of survival for the multiple liver metastases between two groups. CEUS before RFA provided more valuable information for RFA treatment to improve early tumor necrosis and tumor relapse so that patients survival could be prolonged (Figure 7).
The limitations of this study were as follows: (I) Multiple primary sites were included in this study. Different biologic characteristics of primary tumors may influence survival. However, there was no significant difference of primary sites between two groups, and the results of two groups could be comparative. (II) A period effect might be possible because the CEUS group was compared with a historical control group. The control group without CEUS examination was selected from patients with liver metastases treated by RFA before April 2004, while the CEUS group was selected since SonoVue started to be used in our department in May 2004. But all patients underwent the same standard treatment protocols with the same three operators and there was no significant difference in the clinical background of two groups. (III) Because of the complicated condition in clinical practice, we just analyzed the factors of liver metastatic tumor size and number for the therapeutic outcomes after the first RFA. Other therapeutic methods of chemotherapy and transcatheter arterial chemoembolization (TACE) before and after RFA were not analyzed in this study. The general efficacy of a combination of a variety of treatment modalities for liver metastases need further study.
Acknowledgements
This study was supported by Chinese National High Technology Research and Development Program 863 (No. 2009AA02Z4B8) and Project of the Capital Public Health Cultivation (No. Z11110706730000).
Disclosure: The authors declare no conflict of interest.
References
- Stang A, Keles H, von Seydewitz C, et al. Percutanous and intraoperative ultrasound-guided radiofrequency ablation of hepatic tumours. Ultraschall Med 2007;28:181-8.
- Gillams AR, Lees WR. Five-year survival in 309 patients with colorectal liver metastases treated with radiofrequency ablation. Eur Radiol 2009;19:1206-13.
- Meloni MF, Andreano A, Laeseke PF, et al. Breast cancer liver metastases: US-guided percutaneous radiofrequency ablation--intermediate and long-term survival rates. Radiology 2009;253:861-9.
- Chen MH, Wei Y, Yan K, et al. Treatment strategy to optimize radiofrequency ablation for liver malignancies. J Vasc Interv Radiol 2006;17:671-83.
- Wu JY, Chen MH, Yan k, et al. Ultrasound-guided radiofrequency ablation in the treatment of liver metastases. Journal of Beijing Medical University 2001;33:449-51.
- Bernatik T, Seitz K, Blank W, et al. Unclear focal liver lesions in contrast-enhanced ultrasonography--lessons to be learned from the DEGUM multicenter study for the characterization of liver tumors. Ultraschall Med 2010;31:577-81.
- Dai Y, Chen MH, Fan ZH, et al. Diagnosis of small hepatic nodules detected by surveillance ultrasound in patients with cirrhosis: Comparison between contrast-enhanced ultrasound and contrast-enhanced helical computed tomography. Hepatol Res 2008;38:281-90.
- Wilson SR, Burns PN, Muradali D, et al. Harmonic hepatic US with microbubble contrast agent: initial experience showing improved characterization of hemangioma, hepatocellular carcinoma, and metastasis. Radiology 2000;215:153-61.
- Dai Y, Chen MH, Yin SS, et al. Focal liver lesions: can SonoVue-enhanced ultrasound be used to differentiate malignant from benign lesions? Invest Radiol 2007;42:596-603.
- Albrecht T, Blomley MJ, Burns PN, et al. Improved detection of hepatic metastases with pulse-inversion US during the liver-specific phase of SHU 508A: multicenter study. Radiology 2003;227:361-70.
- Oldenburg A, Hohmann J, Foert E, et al. Detection of hepatic metastases with low MI real time contrast enhanced sonography and SonoVue. Ultraschall Med 2005;26:277-84.
- Solbiati L, Goldberg SN, Ierace T, et al. Radio-frequency ablation of hepatic metastases: postprocedural assessment with a US microbubble contrast agent--early experience. Radiology 1999;211:643-9.
- Solbiati L, Tonolini M, Cova L. Monitoring RF ablation. Eur Radiol 2004;14 Suppl 8:P34-42.
- Minami Y, Kudo M, Kawasaki T, et al. Treatment of hepatocellular carcinoma with percutaneous radiofrequency ablation: usefulness of contrast harmonic sonography for lesions poorly defined with B-mode sonography. AJR Am J Roentgenol 2004;183:153-6.
- Solbiati L, Ierace T, Tonolini M, et al. Guidance and control of percutaneous treatments with contrast-enhanced ultrasound. Eur Radiol 2003;13 Suppl 3:N87-90.
- Kim CK, Choi D, Lim HK, et al. Therapeutic response assessment of percutaneous radiofrequency ablation for hepatocellular carcinoma: utility of contrast-enhanced agent detection imaging. Eur J Radiol 2005;56:66-73.
- Chen MH, Yang W, Yan K, et al. The role of contrast-enhanced ultrasound in planning treatment protocols for hepatocellular carcinoma before radiofrequency ablation. Clin Radiol 2007;62:752-60.
- Chen MH, Wu W, Yang W, et al. The use of contrast-enhanced ultrasonography in the selection of patients with hepatocellular carcinoma for radio frequency ablation therapy. J Ultrasound Med 2007;26:1055-63.
- Quaia E, Calliada F, Bertolotto M, et al. Characterization of focal liver lesions with contrast-specific US modes and a sulfur hexafluoride-filled microbubble contrast agent: diagnostic performance and confidence. Radiology 2004;232:420-30.
- Chen MH, Yang W, Yan K, et al. Large liver tumors: protocol for radiofrequency ablation and its clinical application in 110 patients--mathematic model, overlapping mode, and electrode placement process. Radiology 2004;232:260-71.
- Minami Y, Kudo M, Hatanaka K, et al. Radiofrequency ablation guided by contrast harmonic sonography using perfluorocarbon microbubbles (Sonazoid) for hepatic malignancies: an initial experience. Liver Int 2010;30:759-64.
- Gillams AR, Lees WR. Five-year survival following radiofrequency ablation of small, solitary, hepatic colorectal metastases. J Vasc Interv Radiol 2008;19:712-7.
- Sørensen SM, Mortensen FV, Nielsen DT. Radiofrequency ablation of colorectal liver metastases: long-term survival. Acta Radiol 2007;48:253-8.
- Elias D, Baton O, Sideris L, et al. Local recurrences after intraoperative radiofrequency ablation of liver metastases: a comparative study with anatomic and wedge resections. Ann Surg Oncol 2004;11:500-5.
- Bleicher RJ, Allegra DP, Nora DT, et al. Radiofrequency ablation in 447 complex unresectable liver tumors: lessons learned. Ann Surg Oncol 2003;10:52-8.
- Shirabe K, Takenaka K, Gion T, et al. Analysis of prognostic risk factors in hepatic resection for metastatic colorectal carcinoma with special reference to the surgical margin. Br J Surg 1997;84:1077-80.
- Ambiru S, Miyazaki M, Isono T, et al. Hepatic resection for colorectal metastases: analysis of prognostic factors. Dis Colon Rectum 1999;42:632-9.
- Choti MA, Sitzmann JV, Tiburi MF, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg 2002;235:759-66.
- Pawlik TM, Scoggins CR, Zorzi D, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg 2005;241:715-22, discussion 722-4.
- Wernecke K, Rummeny E, Bongartz G, et al. Detection of hepatic masses in patients with carcinoma: comparative sensitivities of sonography, CT, and MR imaging. AJR Am J Roentgenol 1991;157:731-9.
- Glover C, Douse P, Kane P, et al. Accuracy of investigations for asymptomatic colorectal liver metastases. Dis Colon Rectum 2002;45:476-84.
- Albrecht T, Hoffmann CW, Schmitz SA, et al. Phase-inversion sonography during the liver-specific late phase of contrast enhancement: improved detection of liver metastases. AJR Am J Roentgenol 2001;176:1191-8.
- Yin SS, Chen MH, Yan K, et al. Role of gray-scale contrast enhanced ultrasound in diagnosis of liver metastasis. Zhonghua Chao Sheng Ying Xiang Xue Za Zhi (in Chinese) 2005;14:354-8.
- Konopke R, Bunk A, Kersting S. Contrast-enhanced ultrasonography in patients with colorectal liver metastases after chemotherapy. Ultraschall Med 2008;29 Suppl 4:S203-9.
- Solbiati L, Livraghi T, Goldberg SN, et al. Percutaneous radio-frequency ablation of hepatic metastases from colorectal cancer: long-term results in 117 patients. Radiology 2001;221:159-66.


