Bortezomib, dexamethasone plus thalidomide for treatment of newly diagnosed multiple myeloma patients with or without renal impairment
Introduction
Renal impairment is a common feature of multiple myeloma (MM) that may provide a clue to diagnosis and cause a major management problem. Depending on the definition that renal impairment is defined by a serum creatinine (Scr) >2 mg per 100 mL, this complication occurs in 20-40% of newly diagnosed MM patients, with up to 13% having end-stage organ dysfunction requiring dialysis support (1). The major causes of renal impairment are the precipitation of monoclonal light chains in distal and collecting renal tubules and hypercalcemia. Dehydration, hyperuricemia and administration of analgesics and antibiotics with nephrotoxic potential also contribute to its development (2). The median survival of patients with MM has been about 3 years. After the introduction of novel agents such as bortezomib, the prognosis of myeloma patients is steadily improving. The median survival of patients with MM and renal impairment has been less than 2 years, but this figure is likely to be improved with the incorporation of the novel agents including bortezomib (3).
Bortezomib (PS-341, Velcade®; Millenium Pharmaceuticals, Cambridge, MA, USA) is a potent, selective and reversible proteasome inhibitor that has been shown to induce cell death in several tumors including MM (3). The drug has potent anti-myeloma activity in the treatment of both relapsed/refractory and newly diagnosed MM. Adverse events in patients undergoing dialysis are largely similar to those observed in controls and in those with mild-to-severe impairment, with the exception of renal and metabolic adverse events, which were found to be more common in patients undergoing dialysis. Bortezomib-based therapy is also associated with rapid responses typically occurring within the first two cycles of treatment (4). The prognosis of the MM patients with renal impairment may be improved reasonably with the widespread use of bortezomib clinically.
We designed this retrospective analysis to investigate the efficacy and safety of the treatment of the newly diagnosed MM with or without renal impairment receiving the therapy of bortezomib, dexamethasone plus thalidomide (BTD) regimen in order to analyze the effects of BTD regimen on the prognosis of the MM patients with renal impairment compared with the patients without renal impairment.
Materials and methods
Patients
Data were collected from records of 72 newly diagnosed MM patients with or without renal impairment from January 2006 to June 2010. All the patients belonged to International Stage System (ISS) (5) 3 in which transplantation patients were excluded or the patients refused receiving transplantation therapy. According to the level of Scr, the patients were divided into two groups including group 1 (n=42) (Scr <2 mg/dL) and group 2 (n=30) (Scr ≥2 mg/dL).
In both groups, all the patients were treated with BTD regimen as induction therapy. Bortezomib, at a dose of 1.3 mg/m2, was given as an intravenous bolus injection on day 1, 4, 8 and 11. Oral dexamethasone was given at a dose of 40 mg/d on day 1 through 4. Oral thalidomide was given every day at a dose of 200 mg/d. Each cycle was repeated every 21 d for up to 8 cycles. The adverse effects were defined according to a comprehensive grading system for the adverse effects of cancer treatment developed by the National Cancer Institute USA (6). Treatment was withheld if drug-related grade 4 hematological toxic effects or grade 3-4 non-hematological toxic effects occurred. After their resolution, bortezomib could be resumed with a 25% dose reduction (from 1.3 to 1.0 mg/m2 and, if needed, from 1.0 to 0.7 mg/m2). If two or more doses of bortezomib were skipped because of hematological toxicity, a dose reduction of 25% was performed during the following cycle. A 25% dose reduction was required for symptomatic grade 2 neuropathy, whereas bortezomib was interrupted for symptomatic grade 3 neuropathy and resumed with a 50% dose reduction upon complete resolution or reduction of severity to grade 1 neuropathy.
Besides anti-myeloma treatment, all patients received intensive supportive care including intravenous hydration, alkalinization of urine, correction of hypercalcemia and discontinuation of all potential nephrotoxic agents. Renal dialysis was offered to all patients with an appropriate indication. The patients who got partial remission (PR) or above after induction therapy would receive oral thalidomide 200 mg per day as maintenance therapy.
Baseline evaluations included physical examination, blood counts, hepatic and renal function tests, bone marrow aspirate and biopsy, serum and urine protein electrophoreses, and quantitation of serum immunoglobulins and urinary light chains, β2M and C reactive protein. Chest X-ray and complete radiological bone survey were also performed. Safety was assessed throughout the study by physical examinations, recording of vital signs, toxicity assessments, and laboratory tests (hematology, clinical chemistry). All patients that received at least one cycle of BTD regimen were included in the toxicity evaluation.
Statistical analysis
All statistical analyses were performed with SPSS 13.0 statistical software (SPSS Inc., Chicago, IL, USA). The efficacy was evaluated by chi square test. Survival analysis was performed with life table and Kaplan-Meier survival curve. P<0.05 was considered significant for all statistical calculations. Response was evaluated following the International uniform response criteria for multiple myeloma (IMWG) (7). The main indexes included the overall remission (OR), complete remission (CR), very good partial remission (VGPR) and partial remission (PR), in which that OR equals to the summary of CR, VGPR and PR. The renal function was assessed by Kellum et al. reported (8). The reversal of renal function was defined as that the Scr level was decreased and maintained at <133 µmol/L after treatment. The improved renal function was defined as that the Scr level was decreased by 50% or patients treated with hemodialysis did not receive hemodialysis after treatment. Ineffectiveness was defined as the decrease in the Scr level was less than 50% or the hemodialysis continued after treatment.
Results
Patient characteristics
Among these patients, there were 45 males and 27 females with a median age of 62 years (range, 41-82 years). IgG MM was found in 30 patients, IgA in 16 patients, IgD in 6 patients, λ-light chain MM in 6 patients and κ-light chain MM in 14 patients. According to the level of Scr, the patients were divided into two groups including group 1 (Scr <2 mg/dL) (n=42) and group 2 (Scr ≥2 mg/dL) (n=30). Twelve patients in group 2 received hemodialysis at diagnosis. The median Scr level was 3.9 mg/dL (range, 2.2-13.7 mg/dL) in group 2. The baseline patient and disease characteristics were similar at initial diagnosis in the two groups (Table 1).
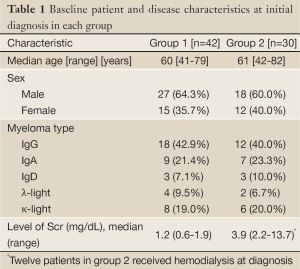
Full Table
Efficacy analysis
All the patients received the therapy of BTD regimen as induction therapy, and the median treatment time was 5 (range, 2-8) cycles. In group 1, 8 patients (19.0%) got CR, 14 (33.3%) VGPR, 12 (28.6%) PR and the OR rate was 81.0%. In group 2, 6 patients (20.0%) got CR, 8 (26.7%) VGPR, 10 (33.3%) PR and the OR rate was 80.0%. There was no statistical difference between the two groups (P>0.05). In group 2, 10 patients (33.3%) got renal function reversal, 14 patients (46.7%) got improved renal function and the median time to renal function reversal was 1.4 months (range, 0.7-3.0 months). Among 12 patients with hemodialysis at diagnosis, 8 patients got rid of hemodialysis after median 4 cycles of therapy (range, 3-6 cycles).
Prognosis
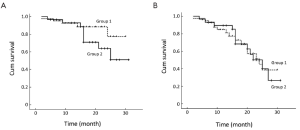
After a median follow-up period of 16 (range, 2-31) months, 5 patients (11.9%) in group 1 died and 9 patients (30.0%) in group 2 died (P=0.056). The 2-year estimate of overall survival was 77.3% in group 1 and 63.8% in group 2, respectively (P=0.188) (Figure 1A).
During a median follow-up time of 13 (range, 2-25) months, 15 patients (35.7%) in group 1 progressed and 13 patients (43.3%) in group 2 progressed (P=0.513). The 2-year estimate of response duration was 50.6% in group 1 and 42.1% in group 2, respectively (P=1.000) (Figure 1B).
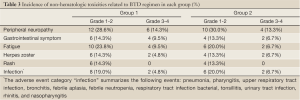
Toxicities related to therapy
All patients experienced at least one adverse event. The main toxicities related to therapy in the two groups included thrombocytopenia, peripheral neuropathy (PN), infection, herpes zoster and so on (Tables 2,3). Most adverse events were National Cancer Institute Common Toxicity Criteria grades 1-2. Thrombocytopenia in two groups was reversible. Once the treatment discontinued, the amount of platelet reached the normal level or the pre-treatment level. Adverse events leading to premature discontinuation of bortezomib were documented in 6 (14.3%) patients of group 1 and 5 (16.7%) patients of group 2. The dose of bortezomib was reduced in 10 patients (23.8%) of group 1 and 8 patients (26.7%) of group 2. No deaths related to therapy were reported in this study.

Full Table
Full Table
Discussion
Renal impairment is one of severe complications of MM. The most common cause is irreversible cast nephropathy secondary to excess serum-free light chains, while, in many instances, renal impairment associated with MM may be linked to additional reversible complications including volume depletion, hypercalcemia, exposure to contrast material, non-steroidal anti-inflammatory drugs, angiotensin converting enzyme inhibitors and tumor lysis syndrome (9).
The mortality in MM patients with acute renal failure is as high as 30% within the initial two months, but that in MM patients with no renal impairment was only 7% (1). Haynes et al. (10) found that the MM patients with renal impairment had a poor prognosis. In addition, if these patients were misdiagnosed as primary renal failure and mistreated for a long time, the probability of renal function reversal was extremely low. Therefore, early, active and effective treatment is crucial for favorable outcomes.
Bortezomib is a proteasome inhibitor and has powerful anti-myeloma activity. It can improve renal function through decreasing the level of toxic monoclonal light chain and suppressing nuclear factor-κb in the tubular cells leading to improved inflammatory response. More recently, serum cystatin-C, a marker of renal impairment and tumor burden was reduced after bortezomib therapy, thus reflecting drug-induced anti-myeloma activity and possibly a direct effect on renal function (11). The toxicity profile of bortezomib in patients with renal impairment was similar to that of cases with no renal impairment (12). In our study, no statistically significant differences in terms of early drug discontinuation rates, or dose reduction, or overall toxicity were observed in patients with or without renal impairment. These results are largely expected because bortezomib pharmacokinetics is independent of renal clearance and is not influenced by the degree of renal impairment. Consequently, different degrees of renal impairment do not require any drug adjustment. This provided the theoretical basis of the therapy with bortezomib for the MM patients with renal impairment.
Bortezomib-based combination treatment with dexamethasone (13), or with the addition of doxorubicin (14) or with melphalan-prednisone (15) has been shown to be highly effective and to rapidly induce tumor response. In our study, the overall response rate was 80.0% in group 2 with BTD regimen therapy, which was similar to the result in group 1. In group 2, 10 patients got renal function reversal, 14 patients got improved renal function and the median time to renal function reversal was 1.4 months. Among 12 patients with hemodialysis at diagnosis, 8 patients got rid of hemodialysis after median 4 cycles of therapy. Ludwig et al. (4) treated the MM patients with light chain-induced acute renal failure (n=68) with bortezomib-doxorubicin-dexamethasone therapy. By intent-to-treat analysis, a myeloma response was obtained in 72% of 18 previously and 50 not previously treated patients, including 38% CR/near CR. Renal response was achieved in 62% of patients with 31% renal CR. Median progression-free survival was 12.1 months, and 1- and 2-year survival rates were 72% and 58%, respectively. Similar results were reported by many authors (16-18).
Whether recovering from renal impairment represents a significant prognostic indicator still remains a controversial issue (19-22). In our study, we found that the MM patients with renal impairment got the same outcome as the MM patients without renal impairment after the therapy with BTD regimen and the two groups had similar prognosis. This proved that bortezomib could overcome the poor prognosis of renal impairment on the MM patients. But the result came from our single center, the number of patients in our study was not enough and the time of follow-up was short. We will further follow up the more patients for a longer time in order to confirm the result.
To summarize, our study along with those of the literature provides evidence for the efficacy and safety of bortezomib-based regimens in the subset of MM patients with renal impairment. BTD regimen may become the front-line therapy for the newly-diagnosed MM patients with renal impairment because BTD regimen can improve the prognosis of the patients with renal impairment as good as the patients without renal impairment.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Augustson BM, Begum G, Dunn JA, et al. Early mortality after diagnosis of multiple myeloma: analysis of patients entered onto the United kingdom Medical Research Council trials between 1980 and 2002--Medical Research Council Adult Leukaemia Working Party. J Clin Oncol 2005;23:9219-26.
- Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc 2003;78:21-33.
- Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood 2008;111:2516-20.
- Ludwig H, Adam Z, Hajek R, et al. Light chain-induced acute renal failure can be reversed by bortezomib-doxorubicin-dexamethasone in multiple myeloma: results of a phase II study. J Clin Oncol 2010;28:4635-41.
- Greipp PR, San Miguel J, Durie BG, et al. International staging system for multiple myeloma. J Clin Oncol 2005;23:3412-20.
- Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 2003;13:176-81.
- Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia 2006;20:1467-73.
- Kellum JA, Hoste EA. Acute kidney injury: epidemiology and assessment. Scand J Clin Lab Invest Suppl 2008;241:6-11.
- Dimopoulos MA, Kastritis E, Rosinol L, et al. Pathogenesis and treatment of renal failure in multiple myeloma. Leukemia 2008;22:1485-93.
- Haynes RJ, Read S, Collins GP, et al. Presentation and survival of patients with severe acute kidney injury and multiple myeloma: a 20-year experience from a single centre. Nephrol Dial Transplant 2010;25:419-26.
- Terpos E, Katodritou E, Tsiftsakis E, et al. Cystatin-C is an independent prognostic factor for survival in multiple myeloma and is reduced by bortezomib administration. Haematologica 2009;94:372-9.
- Jagannath S, Barlogie B, Berenson JR, et al. Bortezomib in recurrent and/or refractory multiple myeloma. Initial clinical experience in patients with impared renal function. Cancer 2005;103:1195-200.
- Jagannath S, Durie BG, Wolf J, et al. Bortezomib therapy alone and in combination with dexamethasone for previously untreated symptomatic multiple myeloma. Br J Haematol 2005;129:776-83.
- Oakervee HE, Popat R, Curry N, et al. PAD combination therapy (PS-341/bortezomib, doxorubicin and dexamethasone) for previously untreated patients with multiple myeloma. Br J Haematol 2005;129:755-62.
- Mateos MV, Hernández JM, Hernández MT, et al. Bortezomib plus melphalan and prednisone in elderly untreated patients with multiple myeloma: results of a multicenter phase 1/2 study. Blood 2006;108:2165-72.
- Ludwig H, Drach J, Graf H, et al. Reversal of acute renal failure by bortezomib-based chemotherapy in patients with multiple myeloma. Haematologica 2007;92:1411-4.
- Roussou M, Kastritis E, Migkou M, et al. Treatment of patients with multiple myeloma complicated by renal failure with bortezomib-based regimens. Leuk Lymphoma 2008;49:890-5.
- Morabito F, Gentile M, Ciolli S, et al. Safety and efficacy of bortezomib-based regimens for multiple myeloma patients with renal impairment: a retrospective study of Italian Myeloma Network GIMEMA. Eur J Haematol 2010;84:223-8.
- Alexanian R, Barlogie B, Dixon D. Renal failure in multiple myeloma. Pathogenesis and prognostic implications. Arch Intern Med 1990;150:1693-5.
- Bladé J, Fernández-Llama P, Bosch F, et al. Renal failure in multiple myeloma: presenting features and predictors of outcome in 94 patients from a single institution. Arch Intern Med 1998;158:1889-93.
- Kastritis E, Anagnostopoulos A, Roussou M, et al. Reversibility of renal failure in newly diagnosed multiple myeloma patients treated with high dose dexamethasone-containing regimens and the impact of novel agents. Haematologica 2007;92:546-9.
- Knudsen LM, Hjorth M, Hippe E. Renal failure in multiple myeloma: reversibility and impact on the prognosis. Nordic Myeloma Study Group. Eur J Haematol 2000;65:175-81.


