Establishment of NOD/SCID mouse models of human hepatocellular carcinoma via subcutaneous transplantation of histologically intact tumor tissue
Introduction
Liver cancer is one of the most common malignancies in the world and the third most common cause of cancer-related death (1,2). The highest age-standardized mortality rate was reported for China (34.7/100,000), which alone accounts for 53% of all liver cancer-related deaths worldwide (3). The incidence of hepatocellular carcinoma (HCC) is slowly but steadily increasing, particularly in the Chinese population, owing to endemic hepatitis B virus (HBV) infection, and in the West, primarily due to increased hepatitis C virus (HCV) infection (4-6). Liver resection and liver transplantation are curative treatments for early-stage HCC. However, HCC is usually diagnosed at an advanced stage accompanied by recurrence and metastasis. Although progress has been made in clinical treatment, overall survival for patients with HCC remains extremely poor (7). Therefore, studies on the mechanism, angiogenesis, progression, recurrence, and metastasis of HCC are of great importance.
Animal models are important tools for investigating the pathogenesis of diseases and developing treatment strategies (8,9). Numerous experimental models have been developed to mimic the characteristics of different stages of HCC in humans and test novel drug candidates (10-13). In the past three decades, a great number of human HCC cell lines have been established (14). In 1963, Chen et al. established the first human HCC cell line, BEL-16. Alexander et al. established the famous human HCC cell line PLC/PRF/5. Dong et al. established the human HCC cell line SMMC-7721 in 1977, and this cell line remains one of the most commonly used human HCC cell lines in China. In 1996, Leveille-Webster et al. established intrahepatic xenografts of human HCC in severe combined immunodeficiency (SCID) mice to study multidrug resistance (15). However, these cell lines and animal models are unable to meet the needs of clinical basic research of HCC, which has very complex etiology such as HBV or HCV infection, aflatoxin B1 (AFB1) exposure and high genetic heterogenicities.
In the present experiment, histologically intact fresh surgical HCC specimens were subcutaneously transplanted into non-obese diabetic/severe combined immunodeficiency (NOD/SCID) mice, which were combined T cell, B cell, and NK cell deficiency. The biological characteristics of the original and corresponding transplanted tumors and cell lines were investigated.
Materials and methods
Mice
Six- to eight-week-old NOD/SCID male and female mice and T cell-immunodeficient BALB/c-nu/nu mice were maintained under standard conditions according to our institution’s guidelines. All animal experiment protocols used in this study were approved by the Shanghai Medical Experimental Animal Care Commission at Shanghai Jiaotong University (approval ID ShCI-12-023).
Surgical human HCC specimens
Fresh surgical specimens were obtained with informed written consent from 24 patients with HCC who had undergone liver cancer resection at the Liver Cancer Institute of Zhongshan Hospital, Fudan University (Shanghai, China). The specimens were rinsed, preserved in ice-cold serum-free Dulbecco’s Modified Eagle’s Medium (DMEM; Sigma-Aldrich, USA), and sent to our laboratory within 2 h.
Subcutaneous transplantation of surgical specimens
After the necrotic and non-cancerous tissues of the specimens were removed, the remaining cancerous tissues were cut into small pieces of approximately 2 mm3 in size, and four or five pieces of tissue were transplanted subcutaneously into the right flanks of NOD/SCID mice. Depending on the surgical specimen’s size, tumor pieces from each patient were generally transplanted into between two and eight mice. Tumor growth was monitored once per week by palpation. Once the subcutaneous tumor reached 10-15 mm in diameter, it was removed and cut into approximately 2 mm × 2 mm × 2 mm pieces, which were transplanted into NOD/SCID mice using the aforementioned method. When the tumor had been passaged three times, it was transplanted into nude mice, and tumor formation and growth were observed. At the same time, the tumor was used to establish an orthotopic transplantation animal model. The subcutaneous tumor was removed and cut into approximately 2 mm × 2 mm × 2 mm pieces, which were transplanted into the left hepatic lobe of the nude mice. Tumor formation was monitored starting 1 week after transplantation. After 6 weeks, all of the mice were sacrificed, and the tumor masses and murine liver tissue samples were dissected by Hematoxylin-eosin (H&E) staining. If a surgical specimen had not grown in NOD/SCID mice after 3 months, it was considered an unsuccessful transplantation.
Primary cell lines and cell culture
The xenografts were removed and used for primary culture in vitro. Primary cell lines of HCC were established by directly cutting tumor fragments into small pieces using microscissors and placed orderly these small pieces on culture dishes. After 4-6 h, DMEM/F12 (Sigma-Aldrich, USA) containing 10% heat-inactivated fetal bovine serum (FBS; Biowest, France), supplemented with 100 IU/mL penicillin G and 100 µg/mL streptomycin (Sigma-Aldrich, USA), were added to the dishes. The medium was routinely changed every 2-3 d. The primary cell cultures were maintained at 37 °C in a 5% CO2 and 95% humidified atmosphere. The cell lines were continuously passaged until mesenchymal cells were no longer apparent. Cell morphology was viewed under light microscope and representative pictures were taken.
Immunocytochemistry
Primary cells were directly cultured on slides. Alpha-fetoprotein (AFP), albumin, cytokeratin 18 (CK18), cytokeratin 19 (CK19), Hep Par 1, fibroblast, Oct4, Notch1, CD133, DLK1 and CD44 were detected by immunocytochemistry using a two-step labeled avidin-biotin immunoperoxidase method, as recommended by the protocol. For the primary antibody dilutions, the concentrations were employed according to Table 1. The results were visualized and photographed using an Axioskop 2 microscope (Carl Zeiss, Germany) with a DP70 CCD system (Olympus, Japan).
Full Table
Cell growth curves
Approximately 1×104 cells were added into each well of a 96-well plate. Cell numbers in three wells were counted in a hemocytometer every 24 h for 7 consecutive days, and cell growth curves were plotted.
Migration and invasion assays
Cell migration and invasion assays were measured by using 6.5-mm Transwell chambers (8 µm pore size; Corning). For invasion assays, 34 µg of Matrigel (BD Biosciences, catalogue No. 354234, MA, USA) was coated onto each filter and the chamber was incubated at 37 °C for 2 h to produce the artificial basement membrane. In both assays, tumor cells in serum-free DMEM (200 µL containing 1×105 cells) were added into the upper compartment of the chamber, and 800 µL of conditioned medium was added to the lower compartment. Plates were incubated at 37 °C in 5% CO2 for 12 h and 24 h for migration assays or 24 h and 36 h for invasion assays. The non-migratory or non-invasive cells were wiped from the upper chambers with cotton wool. The undersides of the chamber were fixed in 100% methanol for 30 min, air-dried, stained with 0.1% crystal violet, and photographed with a CKX41 microscope (Olympus, Japan). Ten random fields were analyzed for each Transwell insert, and the number of cells that had migrated was counted. Assays were conducted for three independent times.
In vivo growth of HCC primary cells
Exponentially growing cells were harvested with trypsin-EDTA and resuspended in FBS-free DMEM. For subcutaneous (s.c.) inoculation, approximately 2×106 cells in 0.2 mL culture medium were injected s.c. into the right flanks of nude mice (n=8), tumor growth was monitored twice per week, and tumor diameters were measured using vernier calipers. The tumor volume was calculated on the basis of the assumption that the tumors were ellipsoid. All eight mice were killed after 4 weeks, and visible tumors were fixed in 10% buffered formalin and processed for H&E staining. Any metastasis to the lungs, liver, kidneys, spleen, or lymph nodes was noted.
Flow cytometry
Exponentially growing cells were harvested, and single-cell suspension containing 1×106 cells was prepared. The cells were treated following a standardized protocol. Molecular markers, including CD133, CD90, EpCAM, CD44, and CD24, which are considered to be cancer stem cell markers, were detected by flow cytometry.
Statistical analysis
Statistical analysis was performed using SPSS version 18.0 (SPSS Inc. Chicago, IL, USA). In this study, quantitative data were expressed as the . Significant differences between the groups were compared using Student’s t-test. P<0.05 was considered statistically significant.
Results
Establishment of patient-like HCC animal models in NOD/SCID mice
Twenty-four surgical HCC specimens were obtained, from which five human HCC animal models were successfully established in NOD/SCID mice by subcutaneous transplantation of histologically intact tumor tissues. The tumorigenicity rate reached 20.83% (5/24). In the first generation, tumors grew relatively slowly with different latency periods ranging from 1 to 3 months (Table 2). However, tumor formation rate was increased and passage of time was gradually shortened with increasing passages (Table 3). The growth of these human HCC models was variable, for example, the volume of one tumor was 2,782.66±1,343.99 mm3 after 7 weeks, whereas that of another tumor reached 7,255.40±2,034.92 mm3 in 6 weeks. One of the five established tumors spontaneously regressed in the third generation, whereas the other four tumors continued to grow for 10 passages in vivo. For orthotopic transplantation, the tumor take rate was 100% (8/8) in nude mice. The resultant tumors closely resembled the original surgical specimens histologically, and they were demonstrated to possess the characteristics of HCC (Figure 1).
Full Table

Full Table
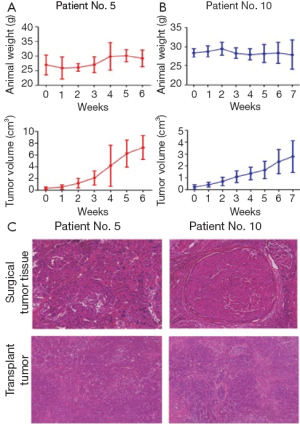
Establishment of primary HCC cell lines
The established subcutaneous xenograft models were used in an attempt to establish primary human HCC cell lines. One specimen was taken from a 65-year-old man with Edmonson grade II HCC. His serum AFP level was 125.6 ng/mL, and he was positive for HBsAg, HBeAg, and HBeAb. Additionally, the patient exhibited extensive intrahepatic and hilar lymph node metastases, but no distant metastases were noted during surgery. The other specimen was obtained from a 56-year-old man with Edmonson grade II HCC. His serum AFP and carcinoembryonic antigen (CEA) levels were 466.9 and 6.48 ng/mL, respectively, and he was positive for HBsAb and HBeAb. Like the other patient, he exhibited extensive intrahepatic and hilar lymph node metastases, but no distant metastases were noted during surgery.
During the first month of primary culture, proliferating tumor cells grew slowly and formed small colonies, some of which were surrounded by a great number of fibroblasts. However, these fibroblasts gradually disappeared as the passage number increased. Two of the four established cell lines could be maintained and continuously cultured in vitro. The resultant two cell lines were established in culture and designated HCC-LY5 and HCC-LY10, respectively. The success rate of culturing primary cell lines was thus 50%. At present, the cell lines have grown continuously for 100 passages and discontinuously for more than 3 years in vitro.
Identification of human HCC cell lines by immunocytochemistry
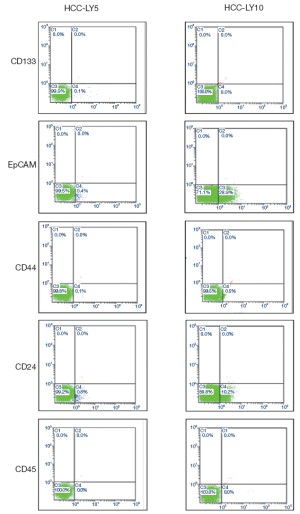
Primary cells were directly cultured on slides, and the expression of molecular markers was detected by immunocytochemistry. The results showed that HCC-LY5 and HCC-LY10 cells were positive for the liver cell-associated proteins albumin, CK18, CK19, and Hep Par1; the liver cancer stem cell-associated proteins Oct4, Notch1, CD90, DLK1 and CD44. HCC-LY5 cells, but not HCC-LY10 cells, expressed CD133 and AFP (Figure 2).
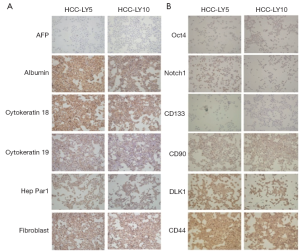
Biological characteristics of primary cell lines
HCC-LY5 and HCC-LY10 cells grew as monolayer polygons with clear boundaries and exhibited a similar morphology. The two cell types were both characterized by an epithelial-like shape with prominent nuclei. The in vitro growth of the two cells was close (P>0.05). For the migration assay, the numbers of cells that penetrated the artificial basement membrane were (126.46±18.07) and (219.13±33.75) cells per high power field at 12 and 24 h, respectively, for HCC-LY5, and (0.63±0.68) and (5.53±1.90) cells per high power field, respectively, for HCC-LY10 (P<0.05). For the invasion assay, the numbers of cells that penetrated the Matrigel were (39.69±27.85) and (74.93±20.12) cells per high power field at 24 and 36 h, respectively, for HCC-LY5, and (5.27±3.41) and (9.22±4.94) cells per high power field, respectively, for HCC-LY10 (P<0.05). Thus, HCC-LY5 cells had higher metastatic potential than HCC-LY10 cells. Primary cultured cells at regular passages were injected subcutaneously into nude mice, and tumor formation was observed. The tumorigenicity of these two primary cell lines reached 100%; the mean latent period was 18 d for both cell lines. The tumor volume was 2,543.84±897.70 mm3 for HCC-LY5 cells and 2,164.91±864.43 mm3 for HCC-LY10 cells. No metastases were identified in nude mice bearing s.c. tumors (Figure 3).
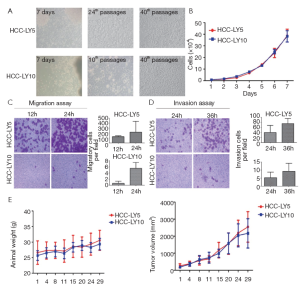
Figure 3 Biological characteristics of the primary cell lines. A. Morphological appearance of each of the established HCC cell lines in culture medium; B. The in vitro growth curves of HCC-LY5 and HCC-LY10 cells. 1×104 cells were seeded in 96-well plates, and cell proliferation was measured by a hemocytometer every 24 h for 7 consecutive days. The results are expressed as ; C. Transwell migration assay in HCC-LY5 and HCC-LY10 cells. 1×105 cells were seeded into Transwell plates and cultured for 12 and 24 h at 37 °C; D. Transwell Matrigel invasion assay in HCC-LY5 and HCC-LY10 cells. 1×105 cells were seeded into Matrigel-coated Transwell plates and cultured for 24 and 36 h at 37 °C. The cells that had invaded or migrated to the underside of the inserts were then stained with crystal violet. The magnification of photograph was 200×. The results are expressed as the
. E. The in vivo growth of primary HCC cell line. 2×106 cells were injected subcutaneously into the right flank of nude mice (n=8), tumor growth was monitored twice per week, and tumor diameters were measured using vernier calipers. Tumor volume=1/2 (length × width × height)
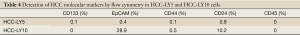
Detection of HCC molecular markers by flow cytometry
A large number of molecular markers have been demonstrated to be associated with cancer stem cells. In this experiment, several molecular markers were detected by flow cytometry. In total, 0.1%, 0.4%, 0.1% and 0.8% of HCC-LY5 cells were positive for CD133, EpCAM, CD44 and CD24, respectively, and 28.9%, 0.5% and 10.2% of HCC-LY10 cells were positive for EpCAM, CD44 and CD24, respectively (Table 4, Figure 4).
Full Table
Discussion
HCC is a prevalent disease worldwide, particularly in China. Patients often die from the recurrence and metastasis of HCC. However, the mechanisms of HCC recurrence and metastasis are poorly understood (16). Liver cancer animal models are important tools for understanding tumor initiation, progression, and metastasis and improving tumor diagnosis, prevention, and treatment. There are four types of HCC models: spontaneous, induced, transplantable, and transgenic/knockout animal models (1,17). Each model has its own advantages and disadvantages. Of these four types, the transplantable animal model is the most widely used. However, the success rate for establishing HCC cell lines is extremely low (16). Compared with other tumor types (18,19), it has been more difficult to establish liver cancer animal models and cell lines. Sun et al. established a highly metastatic model of human HCC in nude mice via the orthotopic implantation of histologically preserved metastatic tumor tissues from 30 surgical specimens, but they reported a transplant success rate of only 3.33% (20,21). To further improve the success rate of tumor transplantation, SCID mice can be used as transplant hosts. In previous research, a total of 326 fresh tumor specimens, primarily gastrointestinal and female genital tissue, were engrafted in NOD/SCID mice, and 54 tissue lines were successfully established (22). Human breast carcinoma animal models were established in SCID mice using intact tissue specimens, and the transplantation success rate was 50% (23). In the present experiment, histologically intact fresh HCC surgical specimens were subcutaneously transplanted in NOD/SCID mice with a greatly improved transplantation success rate of 20.83% (5/24). The histological characteristics of the resulting tumors were similar to those of the original HCC surgical specimens.
Cancer cells obtained from surgical samples can be maintained in primary culture for only a short time. Krause summarized the factors affecting the success of culture growth (24). The highest cause of failure is a lack of adequate viable cells, which are not likely to thrive in vitro. Another complication is the overgrowth of promising tumor cells by fibroblasts. Compared with using surgical samples to directly establish cell lines, a xenograft from an ideal animal model bearing a human tumor would provide more viable cells and a continuous source of live tumor tissue. In the present experiment, we used surgical specimens to directly establish primary HCC cell lines, but no cell lines could be established. However, established subcutaneous xenografts were used in an attempt to establish primary human HCC cell lines. Two of the four established cell lines could be maintained and continuously cultured in vitro, resulting in a 50% success rate. The two primary cell lines were grown continuously for 100 passages and discontinuously for more than 3 years in vitro.
In summary, in vivo and in vitro HCC models and cell lines provide us with a useful platform to study tumor growth and metastasis and preclinically screen antitumor therapies.
Acknowledgements
This work was supported by grants from the National Key Sci-Tech Special Project of China (2008ZX10002-022) and Leading Academic Discipline Project of Shanghai Municipal Education Committee (J50208).
Disclosure: The authors declare no conflict of interest.
References
- Wu L, Tang ZY, Li Y. Experimental models of hepatocellular carcinoma: developments and evolution. J Cancer Res Clin Oncol 2009;135:969-81. [PubMed]
- Tang ZY, Ye SL, Liu YK, et al. A decade’s studies on metastasis of hepatocellular carcinoma. J Cancer Res Clin Oncol 2004;130:187-96. [PubMed]
- Pisani P, Parkin DM, Bray F, et al. Erratum: Estimates of the worldwide mortality from 25 cancers in 1990. Int J Cancer 1999;83:870-73. [PubMed]
- Yau WL, Lam CS, Ng L, et al. Over-expression of miR-106b promotes cell migration and metastasis in hepatocellular carcinoma by activating epithelial-mesenchymal transition process. PLoS One 2013;8:e57882. [PubMed]
- Ochoa-Callejero L, Toshkov I, Menne S, et al. Expression of matrix metalloproteinases and their inhibitors in the woodchuck model of hepatocellular carcinoma. J Med Virol 2013. [Epub ahead of print] [PubMed]
- Liao R, Wu H, Yi Y, et al. Clinical significance and gene expression study of human hepatic stellate cells in HBV related-hepatocellular carcinoma. J Exp Clin Cancer Res 2013;32:22. [PubMed]
- Yang Y, Jin C, Li H, et al. Improved radiosensitizing effect of the combination of etanidazole and paclitaxel for hepatocellular carcinoma in vivo. Exp Ther Med 2012;3:299-303. [PubMed]
- Choufani G, Roper N, Delbrouck C, et al. Animal model for cholesteatoma induced in the gerbil: will the profiles of differentiation/growth-regulatory markers be similar to the clinical situation? Laryngoscope 2007;117:706-11. [PubMed]
- Milane L, Duan ZF, Amiji M. Pharmacokinetics and biodistribution of lonidamine/ paclitaxel loaded, EGFR-targeted nanoparticles in an orthotopic animal model of multi-drug resistant breast cancer. Nanomedicine 2011;7:435-44. [PubMed]
- Jung KH, Zheng HM, Jeong Y, et al. Suppression of tumor proliferation and angiogenesis of hepatocellular carcinoma by HS-104, a novel phosphoinositide 3-kinase inhibitor. Cancer Lett 2013;328:176-87. [PubMed]
- Huang PW, Tsai SC, Luo TY, et al. Therapeutic efficacy of 188Re-MN-16ET lipiodol in an animal model of hepatocellular carcinoma. Ann Nucl Med 2013. [Epub ahead of print] [PubMed]
- Li Y, Tang Z, Ye S, et al. Establishment of human hepatocellular carcinoma cell line with spontaneous pulmonary metastasis through in vivo selection. Zhonghua Yi Xue Za Zhi 2002;82:601-5. [PubMed]
- Li Y, Tian B, Yang J, et al. Stepwise metastatic human hepatocellular carcinoma cell model system with multiple metastatic potentials established through consecutive in vivo selection and studies on metastatic characteristics. J Cancer Res Clin Oncol 2004;130:460-8. [PubMed]
- Saito H, Morizane T, Watanabe T, et al. Establishment of a human cell line (HCC-T) from a patient with hepatoma bearing no evidence of hepatitis B or A virus infection. Cancer 1989;64:1054-60. [PubMed]
- Leveille-Webster CR, Arias IA. Establishment and serial quantification of intrahepatic xenografts of human hepatocellular carcinoma in severe combined immunodeficiency mice, and development of therapeutic strategies to overcome multidrug resistance. Clin Cancer Res 1996;2:695-706. [PubMed]
- Tian J, Tang ZY, Ye SL, et al. New human hepatocellular carcinoma (HCC) cell line with highly metastatic potential (MHCC97) and its expressions of the factors associated with metastasis. Br J Cancer 1999;81:814-21. [PubMed]
- Sánchez A, Fabregat I. Genetically modified animal models recapitulating molecular events altered in human hepatocarcinogenesis. Clin Transl Oncol 2009;11:208-14. [PubMed]
- Merk J, Rolff J, Becker M, et al. Patient-derived xenografts of non-small-cell lung cancer: a pre-clinical model to evaluate adjuvant chemotherapy? Eur J Cardiothorac Surg 2009;36:454-9. [PubMed]
- Fichtner I, Rolff J, Soong R, et al. Establishment of patient-derived non-small cell lung cancer xenografts as models for the identification of predictive biomarkers. Clin Cancer Res 2008;14:6456-68. [PubMed]
- Sun F, Tang Z, Liu K. Growth pattern and metastatic behaviour of orthotopically metastatic model of human hepatocellular carcinoma in nude mice. Zhonghua Yi Xue Za Zhi 1995;75:673-5, 710. [PubMed]
- Sun FX, Tang ZY, Lui KD, et al. Establishment of a metastatic model of human hepatocellular carcinoma in nude mice via orthotopic implantation of histologically intact tissues. Int J Cancer 1996;66:239-43. [PubMed]
- Fujii E, Suzuki M, Matsubara K, et al. Establishment and characterization of in vivo human tumor models in the NOD/SCID/gamma(c)(null) mouse. Pathol Int 2008;58:559-67. [PubMed]
- Visonneau S, Cesano A, Torosian MH, et al. Growth characteristics and metastatic properties of human breast cancer xenografts in immunodeficient mice. Am J Pathol 1998;152:1299-311. [PubMed]
- Rheinwald JG, Beckett MA. Tumorigenic keratinocyte lines requiring anchorage and fibroblast support cultured from human squamous cell carcinomas. Cancer Res 1981;41:1657-63. [PubMed]


