Risk factors associated with early recurrence of adenocarcinoma of gastroesophageal junction after curative resection
Introduction
Adenocarcinoma of the esophagogastric junction (AEG) refers to the tumors located at the transitional zone between esophagus and stomach (1,2). Due to its unique features in terms of epidemiology, genetics, and prognosis, AEG has been regarded as a distinct condition (3). Compared with gastric cancers at other locations, AEG is larger in size, and therefore is more likely to develop infiltration of deep stomach walls, lymph node metastasis, hematogenous metastasis, and early recurrence after surgery (4). Particlurly, its prognosis is poor after radical resection (5-7).
Up to now, many studies have explored the prognostic factors of AEG (8). However, few studies have investigated the recurrence of AEG, particularly, the timing of recurrence. Notably, most AEG patients died of tumor recurrence. Therefore, identifying the risk factors of postoperative recurrence and providing more active treatment for patients at high risk of recurrence will help to improve the prognosis and thus make our previous treatment more meaningful. In our current study, we retrospectively analyzed the clinical data of 147 AEG patients who underwent R0 resection in Beijing Cancer Hospital during the period from December 1995 to December 2007 to identify the relevant factors of early recurrence.
Materials and methods
Subjects
AEG patients who were treated in Beijing Cancer Hospital during the period from December 1995 to December 2007 were enrolled in this study. The inclusion and exclusion criteria are as follows: (I) patients with complete clinical data, and without distant metastasis or other primary tumors according to their preoperative examinations; (II) naive to neoadjuvant chemotherapy before surgery; (III) receiving radical resection in Beijing Cancer Hospital; (IV) with AEG that was pathologically confirmed after surgery; (V) having received complete follow-up; and (VI) patients who died intraoperatively were ruled out; patients who died of surgery-related complications within 90 days after surgery were also ruled out (9). A total of 299 patients were included, among whom 147 (126 males and 21 females, with a median age of 65 years) experienced recurrence. All patients underwent radical resection (D1 in 6 patients and D2 in 141 patients) for AEG. Among them, 63 patients received chemotherapy or radiotherapy after the surgery, whereas no patient had received preoperative chemotherapy or radiotherapy.
Clinical data
Clinical data, including gender, age, body mass index (BMI), T stage, number of positive lymph nodes, Borrmann type, degree of differentiation, vascular tumor thrombi, histological type and postoperative complication, were collected.
Clinicopathological indicators and classification criteria
The conditions were classified as early recurrence (within one year after the first surgery) and late recurrence (one year after the first surgery). According to the anatomic sites, the recurrence was divided as local recurrence (at the anastomotic sites or in the remnant stomach; local surgery field; and in lymph nodes around the abdominal part of the stomach as well as the para-aortic and retroperitoneal lymph nodes), peritoneal metastasis (including Krukenberg’s Tumor, cancer cells detected in the ascites, and peritoneal implantation), hematogenous metastasis (in organs such as liver, bones, and brain), and extra-abdominal metastases. The postoperative disease-free period was defined as the duration from the date of the radical resection to the date on which the symptom, sign, imaging finding, or pathological result of tumor recurrence was identified.
Follow-up
The patients were followed up by phone or mails every three months within two years after surgery, and then every six months, till July 1, 2011. The primary endpoint was mortality.
Statistical analysis
Statistical analysis was performed using SPSS16.0 software (SPSS Inc., Chicago, IL, USA). The differences of the clinical data between these two groups were analyzed with chi-square test. The risk factor of the early recurrence after the surgery for AEG was analyzed using the logistic regression model. The median survival time was calculated using the Kaplan-Meier method, and the survival was compared using Log-rank test. P<0.05 was considered statistically significant.
Results
Time to recurrence
The median follow-up time for this study was 25 months (1-139 months). The mean time to recurrence was 16.3 months, and the recurrence rate within one year was 48.3%.
Sites of recurrence
Among patients with recurrence, peritoneal metastasis was most common (n=52, 35.4%), followed by hematogenous metastasis (n=48, 32.7%), local recurrence (n=42, 28.6%), and extra-abdominal metastases (n=5, 3.4%).
Main risk factors of early recurrence
Degree of differentiation, number of positive lymph nodes, and vascular tumor thrombi showed significantly difference between patients with early recurrence and those with late recurrence (Table 1).
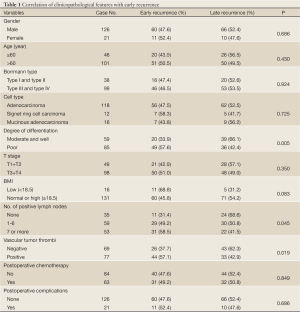
Full Table
Multivariate logistic regression analysis showed that the degree of differentiation and vascular tumor thrombi are the independent risk factors for early postoperative recurrence in AEG patients (Table 2).
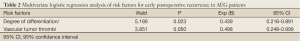
Full Table
Comparison of survival between early recurrence group and late recurrence group
As shown in the survival curve, the median survival time was 38.5 [13-79] months in patients with late recurrence, which was significantly higher than those with early recurrence 14.0 [1-73] months (Figure 1).
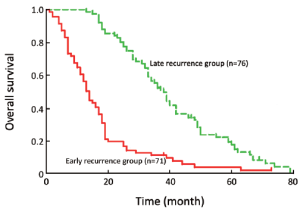
Discussion
Surgery remains to be its main treatment for AEG. However, the postsurgical complications are common and occur within short intervals, with a mean time to recurrence of only 1 year (10,11). Also, the prognosis of patients with late recurrence is better than those with early recurrence. Obviously, early recurrence after surgery is a key factor that affects survival. While the risk factors of the early recurrence after the radical resection for AEG remain controversial, it is basically accepted that whether R0 can be achieved is an important factor (12). Therefore, in our current study, we analyzed the risk factors of early recurrence in patients for whom R0 resection was achieved, with an attempt to provide more active treatment.
Some previous studies concluded that the decreased body weight might result in the poor prognosis in AEG patients (13,14); similarly, our study found that patients with a low BMI tended to develop early recurrence (P=0.083). This may be explained by the fact that a low BMI often represents that the tumor was already in a more advanced stage, and nutritional deficiency can also cause the decrease of the immune function. However, whether BMI is an independent risk factor for the early recurrence still needs further investigation.
Multivariate logistic regression analysis showed that the degree of differentiation and vascular tumor thrombi are the independent risk factors for early postoperative recurrence in AEG patients. Tumor cells that are poorly or undifferentiated can also increase the risk of lymph node metastasis (15); also, vascular tumor thrombus is closely related with lymph node metastasis, suggesting a poor prognosis (16). In our study, the number of positive lymph nodes was a risk factor for early recurrence in the univariate analysis but showed no statistical significance in the multivariate analysis. This may be because the number of positive lymph nodes is closely associated with vascular tumor thrombi and the degree of differentiation. During the surgery, standard D2 radical resection should be applied, and the removal of more than 14 lymph nodes will be helpful to lower the early recurrence rate.
Due to the long research period, although D2 radical resection was applied in 95.9% of patients, the dissected lymph nodes were less than 15 in 79 patients (53.7%). In patients in whom less than 15 or more than 15 lymph nodes were removed, the mean number of positive lymph nodes was 3.3 and 5.8, respectively (rank means: 55.94 vs. 94.99) (P<0.001), suggesting that statistical bias can result from the insufficient removal of lymph nodes. In addition, some tiny recurrent lesions may be neglected in clinical settings, which may also affect the research findings. Also, the median age of our subjects was up to 65 years. During the research, 23 patients died of other causes such as diabetes and hypertension and therefore were ruled out. Although our follow-up was quite timely within one year after surgery, the follow-up was less frequent after one year, which may also lead to bias. Therefore, the high early recurrence rate (48.3%) in our study might be somehow exaggerated.
Some authors have argued that a second surgery may be performed after the local recurrence; the palliative resection has an efficacy comparable to that of conservative therapy, whereas the radical resection is much superior to them (17). Therefore, if the chance for radical resection still exists after the postoperative recurrence, active surgery should be performed to prolong the survival. Even if radical resection becomes unfeasible, palliative resection should be conducted in feasible patients to alleviate or reduce symptoms (e.g., bleeding, obstruction, or cachexia) caused by the primary tumor and improve the quality of life.
In conclusion, histological grade and vascular invasion are independent factors for predicting the tumor recurrence after R0 resection for AEG.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Herbella FA, Patti MG, Takassi GF. Skin metastases from esophageal and esophagogastric junction cancer. J Gastrointest Oncol 2011;2:104-5. [PubMed]
- Zhao Q, Li Y, Yang PG, et al. The application of modified double tracks anastomosis in patients with adenocarcinoma of the esophagogastric junction treated with radical gastrectomy. Transl Gastrointest Cancer 2013;2:AB35.
- Tepper JE, O'Neil B. Transition in biology and philosophy in the treatment of gastroesophageal junction adenocarcinoma. J Clin Oncol 2009;27:836-7. [PubMed]
- Zhang XD, Shu YQ, Liang J, et al. Combination chemotherapy with paclitaxel, cisplatin and fluorouracil for patients with advanced and metastatic gastric or esophagogastric junction adenocarcinoma: a multicenter prospective study. Chin J Cancer Res 2012;24:291-8. [PubMed]
- Ohno S, Tomisaki S, Oiwa H, et al. Clinicopathologic characteristics and outcome of adenocarcinoma of the human gastric cardia in comparison with carcinoma of other regions of the stomach. J Am Coll Surg 1995;180:577-82. [PubMed]
- Saito H, Fukumoto Y, Osaki T, et al. Distinct recurrence pattern and outcome of adenocarcinoma of the gastric cardia in comparison with carcinoma of other regions of the stomach. World J Surg 2006;30:1864-9. [PubMed]
- Casson AG, Darnton SJ, Subramanian S, et al. What is the optimal distal resection margin for esophageal carcinoma? Ann Thorac Surg 2000;69:205-9. [PubMed]
- Siewert JR, Feith M. Adenocarcinoma of the esophagogastric junction: competition between Barrett and gastric cancer. J Am Coll Surg 2007;205:S49-53. [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [PubMed]
- Hulscher JB, van Sandick JW, Tijssen JG, et al. The recurrence pattern of esophageal carcinoma after transhiatal resection. J Am Coll Surg 2000;191:143-8. [PubMed]
- Mariette C, Balon JM, Piessen G, et al. Pattern of recurrence following complete resection of esophageal carcinoma and factors predictive of recurrent disease. Cancer 2003;97:1616-23. [PubMed]
- Lerut T, Moons J, Coosemans W, et al. Postoperative complications after transthoracic esophagectomy for cancer of the esophagus and gastroesophageal junction are correlated with early cancer recurrence: role of systematic grading of complications using the modified Clavien classification. Ann Surg 2009;250:798-807. [PubMed]
- Polee MB, Hop WC, Kok TC, et al. Prognostic factors for survival in patients with advanced oesophageal cancer treated with cisplatin-based combination chemotherapy. Br J Cancer 2003;89:2045-50. [PubMed]
- Stahl M, Wilke H, Stuschke M, et al. Clinical response to induction chemotherapy predicts local control and long-term survival in multimodal treatment of patients with locally advanced esophageal cancer. J Cancer Res Clin Oncol 2005;131:67-72. [PubMed]
- Rice TW, Zuccaro G Jr, Adelstein DJ, et al. Esophageal carcinoma: depth of tumor invasion is predictive of regional lymph node status. Ann Thorac Surg 1998;65:787-92. [PubMed]
- von Rahden BH, Stein HJ, Feith M, et al. Lymphatic vessel invasion as a prognostic factor in patients with primary resected adenocarcinomas of the esophagogastric junction. J Clin Oncol 2005;23:874-9. [PubMed]
- Schipper PH, Cassivi SD, Deschamps C, et al. Locally recurrent esophageal carcinoma: when is re-resection indicated? Ann Thorac Surg 2005;80:1001-5; discussion 1005-6.[PubMed]


