Primary thymic mucosa-associated lymphoid tissue lymphoma with multiple thin walled lung cysts: case report and literature review
Introduction
Mucosa-associated lymphoid tissue (MALT) lymphoma of the thymus is rare. In 1990, Isaacson et al. first described two cases of primary low-grade thymic lymphoma, both of which had histological and immunophenotypic features of extranodal B-cell lymphoma of MALT, or marginal zone lymphoma (MZL) of MALT as proposed in the World Health Organization classification (1). We described a case of low-grade thymic B-cell lymphoma in order to review the pathologic and clinical features of the reported cases in the literature.
Case report
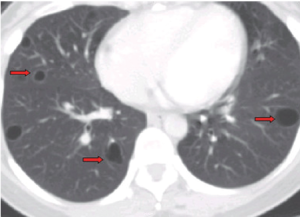
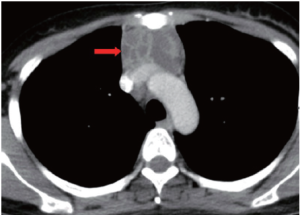
A 37-year-old woman presented to our hospital after 3 weeks of cough without sputum. The symptom progressed and aggravated while lying down. Physical examination revealed grossly normal. Chest radiograph revealed an anterior mediastinal mass lesion. Chest computed tomography (CT) demonstrated a multiloculated cystic mass, 9.5 cm × 5.3 cm × 2.6 cm in mediastinum prevascular space, closely contacting to adjacent vascular structure, especially brachiocephalic vein and superior vena cava (SVC) (Figure 1). Multiple thin-walled cysts in bilateral lungs were noted (Figure 2).
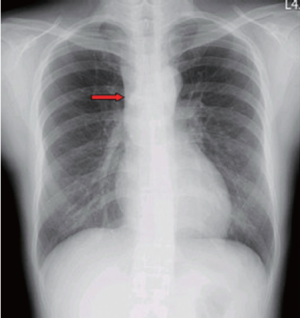
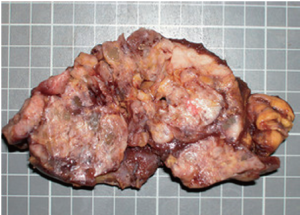
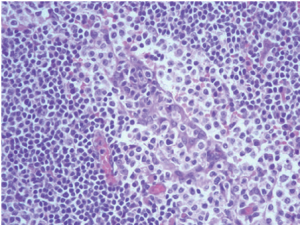
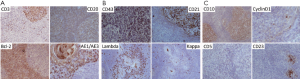
Chest plain film showed a mediastinum mass (Figure 3). A maximal thymectomy with lymph node dissection via median sternotomy revealed a tumor about 11.8 cm × 6.2 cm × 3.1 cm at thymus with multiple enlarged lymph nodes (Figure 4). The pathology diagnosis was confirmed with thymic extranodal marginal zone B-cell lymphoma of MALT. The thymic extranodal marginal zone B-cell lymphoma of MALT is featuring a proliferation of small irregular monocytoid B cells around and between reactive lymphoid follicles, with extensive invasion of the Hassal corpuscles and the thymic epithelium lining the cystic spaces, forming lymphoepithelial lesions (Figure 5). Under immunohistochemical stains, the neoplastic cells are positive for CD20 and Bcl-2; negative for CD3, CD5, CD23, and CD10 (Figure 6). The plasma cells showed monotypic Ig light chain (Lambda type). The pathology report of the excised regional lymph nodes was reactive hyperplasia.
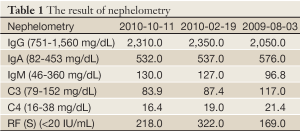
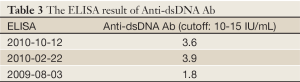
Autoimmune markers were positive for antinuclear antibody (ANA), Sjögren’s syndrome A (SSA), Sjögren’s syndrome B (SSB), and rheumatoid factors (RF), and the elevation of total IgG, IgA and IgE was noted 2 weeks after operation. ANA, anti-SSA and anti-SSB were tested via immunofluorescence test. There were no obvious changes of IgG and IgA 14 months later (Tables 1-4). A bone marrow biopsy obtained 4 months after operation was negative. Sicca symptoms were noted. Shirmer test was 2 and 7 mm. The diagnosis of primary Sjögren’s syndrome was then confirmed (2).
Full Table

Full Table
Full Table
Full Table
Discussion
MALT lymphoma of the thymus is rare. Most patients were Asians and associated with autoimmune disease, especially Sjögren’s syndrome. Racial and environmental factors may influence its development (3).
In our case, immunoglobin and antibody abnormalities remain unchanged one half years after thymectomy. Shimizu et al. thought that immunologic disorders are strongly associated with thymic MALT lymphoma tumorigenesis and that thymic MALT lymphoma was not the cause of immunologic disorders (4).
Thymic MALT lymphoma is often presented with macroscopic and microscopic cyst. Cyst formation may be related to the tendency for cystic transformation of medullary duct-epithelium-derived structures (including Hassall’s corpuscles) when tumor grows in the thymic gland (5). Thymic MALT lymphoma needs to be included in the differential diagnosis of Asian patients with thymic masses accompanied by cystic changes and autoimmune diseases (4).
In patient with Sjögren’s syndrome, pulmonary cysts on high-resolution CT (HRCT) scan, isolated or associated with lymphocytic interstitial pneumonitis or lymphoma, may be due to a peribronchiolar infiltration leading to sequelae of fibrosis and a thin walled cyst (6). Our case was presented with Sjögren’s syndrome and the chest CT revealed bilateral, multiple lung thin walled cysts which had progression in the follow-up period. Differential diagnosis should include lymphangioleiomyomatosis.
Elevation of IgA and IgG was presented in our case (Table 1). This IgA expression discriminates thymic MALT lymphoma from nonthymic MALT lymphomas, which typically express IgM, and less often IgG or IgA (3). Therefore, in Asian patients with thymic masses accompanied by cystic changes and autoimmune disease (hyperglobulinemia), thymic MALT lymphoma should be considering (4). Bcl-2 expression is noted only in small neoplastic cells but negative in large cell transformation, like our case (Figure 6A).
Currently, there are no standard therapeutic protocols or guidelines for the treatment of thymic MALT lymphoma. Surgery, chemotherapy, or radiotherapy alone, or in combination has been commonly used. Most patients were reported in good results. In our case, maximal thymectomy with lymph node dissection was performed. Adjuvant therapy was not prescribed due to non-extrathymic involvement.
In conclusion, the presented case is a Chinese woman with Sjögren’s syndrome and elevation of IgA and IgG. Thymic MALT lymphoma with macroscopic and microscopic cysts was identified and surgical resection was done. Multiple lung cysts with progression were noted. No evidence of recurrence of disease was found after 2 years follow-up.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Isaacson PG, Chan JK, Tang C, et al. Low-grade B-cell lymphoma of mucosa-associated lymphoid tissue arising in the thymus. A thymic lymphoma mimicking myoepithelial sialadenitis. Am J Surg Pathol 1990;14:342-51. [PubMed]
- Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis 2002;61:554-8. [PubMed]
- Inagaki H, Chan JK, Ng JW, et al. Primary thymic extranodal marginal-zone B-cell lymphoma of mucosa-associated lymphoid tissue type exhibits distinctive clinicopathological and molecular features. Am J Pathol 2002;160:1435-43. [PubMed]
- Shimizu K, Yoshida J, Kakegawa S, et al. Primary thymic mucosa-associated lymphoid tissue lymphoma: diagnostic tips. J Thorac Oncol 2010;5:117-21. [PubMed]
- Chan J. Tumors of the lymphoreticular system, including spleen and thymus. In: Fletcher CDM. eds. Diagnostic histopathology of tumors. 2nd ed. London: Churchill Livingstone, 2000:1276-86.
- Hatron PY, Tillie-Leblond I, Launay D, et al. Pulmonary manifestations of Sjögren’s syndrome. Presse Med 2011;40:e49-64. [PubMed]