Allogeneic hematopoietic stem cell transplantation for patients with acute leukemia
Introduction
Over the past nearly 30 years, the chemotherapy has been the most common and guideline-recommended first-line treatment modality for acute leukemia (AL) in young patients (1,2). Although standard induction chemotherapy results in complete remission (CR) in 60% to 80% of the young AL patients, relapse will occur in majority of them within months if no potent post-remission treatment is given to eradicate residual leukemic cells. Especially in patients with relapsed, refractory, or other high-risk AL, long-term disease-free survival (DFS) is rare even after achieving a remission to high-dose chemotherapy (3-5). Therefore, how to improve the long-term survival rate of AL patients is still a critical issue (6,7).
Allogeneic hematopoietic stem cell transplantation (HSCT) that includes allogeneic bone marrow transplantation (BMT), allogeneic peripheral blood stem cell transplantation (PBSCT) and cord blood transplantation (CBT), has become an important approach for treatment of a range of blood diseases as well as some solid tumors and immunodeficiency disorders (8-10). Nowadays, allogeneic HSCT is considered the only curative therapy for AL, because of its distinct advantage of the graft-versus-leukemia (GvL) effect. However, several problems remain to be further determined, such as the selection of the suitable candidates for HSCT, optimal sources of donors, and timing for the procedure as well as preparative regimen, and how to overcome the transplant rejection or graft versus host disease (GvHD), and control or avoid the transplant-related complications (11-13). Here, we reviewed 93 AL patients less than 60 years old (median age, 30 years) who underwent allogeneic HSCT during the past 12 years in Xiangya hospital, for assessing the efficacy of allogeneic HSCT in these patients and analyzing the influential factors of prognosis.
Patients and methods
Patients
Between July 2000 and March 2012, a total of 93 AL patients underwent allogeneic HSCT in our hospital, of whom 57 cases were males and 36 were females with a median age of 30 years (range, 8-46 years). Diagnosis was based on MICM (morphology, immunology, cytogenetics, and molecular biology) typing standard, and among the patients, 62 cases were acute myeloid leukemia (AML) that was composed of AML-M0 (1 case), AML-M1 (8 cases), AML-M2 (29 cases), AML-M4 (16 cases), AML-M5 (6 cases) and AML-M6 (2 cases), 18 cases were acute lymphoblastic leukemia (ALL), 7 cases were acute hybrid leukemia (AHL), 3 cases were myelodysplastic syndromes and refractory anemia with excess of blasts in transformation (MDS-RAEBt), and 3 cases were AL transformed from MDS (MDS-AL). The patients had no other serious complications involving the major organs before transplantation, and were durable for high-dose radiotherapy and chemotherapy. Patients were regarded with high-risk AL when any of the following conditions applied (14): (I) Refractory AL; (II) AL beyond the first remission phase; (III) AL with cytogenetic markers for poor prognosis such as positive Philadelphia chromosome (Ph+) and complex karyotype (3 or more chromosomal abnormalities); (IV) MDS-RAEBt or MDS evolving to AL.
Conditioning regimens
Most AML patients received the BAC regimen (busulfan 0.8 mg/kg/q 6 h ×16 doses, cytarabine 2.0-2.5 g ×2 doses and cyclophosphamide 1.8 g/m2 ×2 doses; most ALL patients and those with central nervous system leukemia were treated with the TBI + CY regimen (8.0-10.0 Gy total body irradiation and cyclophosphamide 1.8 g/m2 daily for 2 days). In a few cases, the regimens were adjusted based on the above classical regimens.
Graft type and stem-cell source
Among the 93 patients, 91 cases underwent PBSCT, and 1 case each received BMT and CD34-selected PBSCT; 60 cases had sibling (related) donor transplantation, and the other 33 cases were transplanted from unrelated donors; 73 cases received human leucocyte antigen (HLA)-matched transplantation, and 20 cases had HLA-unmatched transplantation; 54 cases had ABO compatible donors, and 39 cases were incompatible with their donors.
Prophylaxis and management of GvHD
The prominent regimen for GvHD prophylaxis consisted of cyclosporine A (CsA) and short course of methotrexate, and anti-lymphocyte globulin and mycophenolate mofetil (MMF) were additionally administered in unrelated-HSCT or HLA-unmatched related-HSCT subjects. The treatment regimen for GvHD consisted of CsA, prednisone, and short-term methotrexate, with the addition of tacrolimus, mycophenolate mofetil or monoclonal antibodies depending on the individual’s specific condition. The diagnosis and grading of GvHD were done according to established criteria (15), and grade III-IV acute GvHD (aGvHD) was termed severe aGvHD.
Engraftment, chimerism and minimal residual disease detection
Engraftment was defined as the first of 3 consecutive days with white blood cell (WBC) count ≥1.0×109/L and neutrophil count ≥0.5×109/L. Platelet engraftment was defined as the first of 7 consecutive days that the platelet count ≥20×109/L without transfusion support. Chimerism analysis was performed through fluorescent in situ hybridization (FISH) studies in sex mismatched cases for Y chromosome, and DNA microsatellite polymorphisms, etc. Minimal residual disease detection was performed by means of FISH, reverse transcriptase PCR, flow cytometry or other methods as appropriate.
Treatment of post-transplant relapse
Treatment of post-transplant relapse was heterogeneous and varied depending on patient’s condition, which included immunosuppressant withdrawal, chemotherapy, radiotherapy, intrathecal injection of chemotherapeutic agents, modulating immunity, and donor HSC or lymphocyte infusions.
Statistical analysis
All patients except one were followed up until September 30, 2012. The cumulative incidence of transplantation related mortality (TRM), relapse rate, 3-year overall survival (OS) and 3-year DFS were calculated by Kaplan-Meier method. In univariate analysis, χ2 test and Fisher’s exact test were used to assess differences between the groups, and those variables with P less than 0.10 in univariate analysis were considered for inclusion in multivariate Cox regression models. P values less than 0.05 were considered as statistically significant. All statistical analyses were conducted with SPSS software (version 19.0, SSPS Inc, Chicago, IL).
Results
Hematopoietic reconstitution
The median number of mononuclear cells (MNC) infused was 7.85×108/kg (range, 5.00×108-11.70×108/kg) and CD34+ cells infused was 2.80×106/kg (range, 0.50×106-7.100×106/kg), respectively. Hematopoietic reconstitution was achieved in 90 patients with an engraftment rate of 96.77%. The medial time to WBC count >1.0×109/L was 13 days (range, 9-15 days) and to neutrophil count >0.5×109/L was 12 days (range, 9-21 days), and to achieve a platelet count ≥20×109/L without transfusion was 16 days (range, 10-35 days) after transplantation, respectively.
Transplant-related complications
The incidence of lung infection within the first 100 days post-transplantation was 43.0%, and the incidence of mixed lung infection (bacteria and fungi) was 26.5%. Six patients with lung infection died. In patients having a history of pulmonary fungal infection before transplantation, the incidence of mixed lung infection within the first 100 days post-transplantation was 50.1%.
Thirty-eight patients developed aGvHD, and 13 cases of them were severe aGvHD with 8 death. Eighty-five patients survived more than 100 days, and chronic GvHD (cGvHD) occurred in 37 cases (43.5%), 17 cases (20%) of whom were extensive cGvHD. As showed by the risk factor analysis, the incidences of severe aGvHD in patients ≥40 years of age, and with HLA mismatched grafts or severe lung infection within the first 100 days after transplantation were significantly higher than those in their paired controls (all P<0.05), and the incidences of extensive cGvHD in patients ≥40 years of age, with severe lung infection within the first 100 days after transplantation and having a history of severe aGvHD were significantly higher than those in their paired controls (all P<0.05) (Table 1).
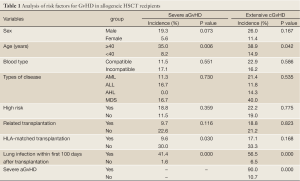
Full Table
Survival, TRM and relapse
The median follow-up period was 26 months (range, 0.1-138 months). At the last follow-up of this study, 63 patients remained alive and 30 patients died. In the entire group of the 93 patients, the 3-year OS and DFS after allogeneic HSCT were 64.6%±5.4% (95% CI, 55.8-76.4%) and 56.54%±5.51% (95% CI, 47.2-66.5%), respectively. One AML patient survived for the length of this study (138 months) after transplantation. The 3-year OS and DFS for AML patients were 70.7%±6.2% (95% CI, 57.6-92.4%) and 61.9%±6.6% (95% CI, 49.8-72.3%), for ALL patients were 47.4%±13.7% (95% CI, 35.6-54.9%) and 35.7%±12.0% (95% CI, 28.7-39.4%), respectively. Of the 7 AHL patients, 2 cases died due to relapse and 1 case died of aGvHD; Of the 6 MDS patients, 1 case each died of relapse and aGvHD.
TRM occurred in 16 patients, of whom, 1 AML patient failed to achieve hematopoietic reconstitution and died 2.5 months after transplantation from a severe pulmonary infection, 2 AML patients died of heart failure during the preparative regimen for transplantation, 7 cases died of severe aGvHD with severe pulmonary infection, 4 cases died of severe pulmonary infection, 1 case died of severe aGvHD, and 1 case died of liver failure. The 3-year cumulative incidence of TRM was 16.8%±6.1% (95% CI, 13.8-17.4%). Univariate analysis indicated that the incidences of TRM in patients ≥40 years, with HLA mismatched graft, severe lung infection within the first 100 days after transplantation, and severe aGvHD were significantly increased versus their paired controls (all P<0.05), while the variables such as sex, blood relationship, type of disease and high-risk AL were not detectably associated with incidence of TRM (all P>0.05).
Twenty-one patients (10 AML, 8 ALL, 2 AHL and 1 MDS-AL) relapsed by the end of follow up, and the 3-year cumulative incidence of relapse was 21.3%±6.7% (95% CI, 18.6-23.5%). Among these patients, 9 cases developed extra-medullary relapse, and 12 cases developed hematological relapse. Six cases of the 9 patients with extra-medullary relapse remitted again and were still alive without disease after radiotherapy, intrathecal injection of chemotherapeutic agents, immunomodulator (IL-2) administration or donor lymphocyte infusion, while the remaining 3 cases died from hematological relapse, and 2 cases of the 12 patients with hematological relapse survived after chemotherapy and donor lymphocyte or stem cells infusion. Analysis of risk factors for relapse found that patients with high-risk AL or lack of cGvHD had a significantly higher relapse rate compared with their paired controls (both P<0.05), and patients who developed extensive cGvHD presented a lower chance of relapse than those who did not develop this condition, but the difference did not reach statistical significance (P=0.102) (Table 2).
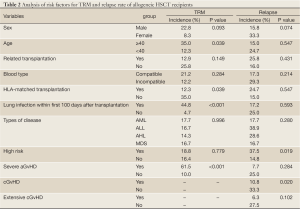
Full Table
Prognostic factors
The differences among stratified variables in 3-year DFS were analyzed for determination of the risk factors responsible for poor outcomes following allogeneic HSCT. Univariate analysis indicated that HLA mismatch, severe lung infection within the first 100 days post-HSCT, high risk AL, severe aGvHD and lack of cGvHD were associated with unfavorable results, and further multivariate analysis identified that severe lung infection within the first 100 days post-HSCT, severe aGvHD and lack of cGvHD were independent risk factors for poor prognosis (Table 3).
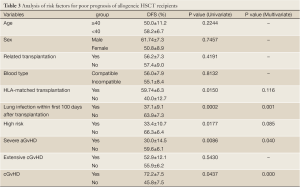
Full Table
Discussion
In this report, we have analyzed 93 patients with AL undergoing allogeneic HSCT over the past 12 years in our hospital. The results showed that the 3-year OS and DFS for the entire group of patients were 64.6%±5.4% and 56.5%±5.5%, for AML patients (with the exclusion of M3 patients) were 70.6%±6.1% and 61.9%±6.6%, and for ALL patients were 47.4%±13.7% and 35.7%±12.0%, respectively. These results suggested that allogeneic HSCT is an effective treatment modality for patients with AML and ALL as previously reported (3,16-20), but could not draw a significant conclusion for AHL and MDS due to the small number of such cases in this study, which requires verification with a larger sample.
TRM is an important cause of allogeneic HSCT failure (21-23). In our data, the proportion of TRM in the total death-rate was 53.3% (16/30), for which the main causes of death were severe aGvHD and/or serious lung infection (12/16). As shown in the analyses, age ≥40 years, HLA mismatch, severe lung infection within the first 100 days post-transplant, and severe aGvHD were significantly associated with higher incidence of TRM, and as well, severe lung infection within the first 100 days post-transplant, and severe aGvHD were independent risk factors for poor prognosis of the patients undergoing allo-HSCT. Thus, how to aggressively control severe aGvHD and decrease early lung infection are critical to reducing the incidence of TRM.
As determined by analysis of the relevant factors affecting aGvHD, the incidences of severe aGvHD were increased in patients ≥40 years of age, and with HLA mismatched transplantation or severe lung infection within the first 100 days post-transplantation versus their paired controls (all P<0.05). In our hospital, the incidence of severe lung infection during the first 100 days post-transplantation in patients having a history of pulmonary fungal infection before transplantation was 50.1%, and the mortality rate in patients developing severe lung infection was 44.8%, which was only 4.7% in their controls. And not only that, but the incidence of simultaneous severe aGvHD in patients with severe lung infection was high (41.4%). As for the reason, the following three points are considered: (I) Benefits of using massive doses of chemotherapy drugs to reduce the residual tumor cells before transplantation is accompanied with the risk of infection, as evidenced by the increased incidence of severe respiratory infections in recent years. And secondly, patients with pulmonary fungal infection require a long course of therapy, and trend to recur when the immune response decline; (II) Various immunosuppressants are often used for prophylaxis of rejection in patients receiving HLA mismatched transplantation; (III) Infection may elicit GvHD, and the severe aGvHD cases usually require even enhanced immunosuppression, which in turn increases the risk of infections and thereby initiates a vicious circle. Thus, we should fully evaluate the patients to determine the risks and benefits of transplantation, and then to decide whether the transplantation can be done or not, as well as the timing and type of transplantation. Meanwhile, solutions to eliminate the unfavorable conditions should be introduced as complete as possible before transplantation.
Relapse is another major cause responsible for allogeneic HSCT failure (24,25). In our series, the proportion of death from relapse accounted for 43.3% (13/30) of the total death-rate; patients with high-risk AL or lack of cGvHD had a higher relapse rate compared with their paired controls, while the severe aGvHD, and HLA mismatched or unrelated transplantation provided no benefit for preventing relapse. Furthermore, multivariate analyses identified that lack of cGvHD was an independent risk factor for poor prognosis. GvL is a major beneficial effect of allogeneic HSCT, but it is always closely linked to GvHD. In clinical practice, powerful immunosuppressants are often used for prevention of occurrence of severe GvHD after transplantation, which in turn may compromise the GvL effect and result in an increased relapse rate. Data from our hospital showed that the relapse rate in patients experienced cGvHD was significantly lower than in those without cGvHD (10.8% vs. 33.3%, P<0.05), and patients with extensive cGvHD also had a lower chance of relapse than those without this condition but no statistical significance was reached (6.3% vs. 27.5%, P>0.05), and this fact indicates that the limited GvHD offers the advantages of not only reducing relapse but also improving quality of life of allogeneic HSCT patients. So, the treatment plans for the patients should be gradated and individualized. And in patients with high-risk factors, both dose and course of immunosuppressive drugs should be reduced as much as possible on the basis of severe aGvHD prophylaxis, and immunoregulatory measures such as immunomodulator regimens and donor lymphocyte infusion should be implemented as early as possible to induce moderate GvHD which produces the GvL effect.
In conclusion, allogeneic HSCT is the main and even the only choice for treatment of AL, and whether there is therapeutic benefit depends on the comprehensive assessment for the disease severity and potential risks associated with transplantation. Further development and improvement of HSCT techniques along with gradated and individualized therapy are expected to provide better outcomes and improved quality of life for AL patients.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Advani AS, Hunger SP, Burnett AK. Acute leukemia in adolescents and young adults. Semin Oncol 2009;36:213-26. [PubMed]
- Styczynski J. A step forward in acute lymphoblastic leukemia. Transl Pediatr 2012;1:68-9.
- Phillips GL. Allogeneic hematopoietic stem cell transplantation (HSCT) for high-risk acute myeloid leukemia (AML)/myelodysplastic syndrome (MDS): how can we improve outcomes in the near future? Leuk Res 2012;36:1490-5. [PubMed]
- Miyawaki S. Clinical studies of acute myeloid leukemia in the Japan Adult Leukemia Study Group. Int J Hematol 2012;96:171-7. [PubMed]
- Liew E, Atenafu EG, Schimmer AD, et al. Outcomes of adult patients with relapsed acute lymphoblastic leukemia following frontline treatment with a pediatric regimen. Leuk Res 2012;36:1517-20. [PubMed]
- Prasanna PG, Stone HB, Wong RS, et al. Normal tissue protection for improving radiotherapy: Where are the Gaps? Transl Cancer Res 2012;1:35-48. [PubMed]
- Servais S, Baron F, Beguin Y. Allogeneic hematopoietic stem cell transplantation (HSCT) after reduced intensity conditioning. Transfus Apher Sci 2011;44:205-10. [PubMed]
- Platzbecker U. Allogeneic hematopoietic cell transplantation in patients with myelodysplastic syndromes. Semin Hematol 2012;49:342-9. [PubMed]
- Cassaday RD, Gopal AK. Allogeneic hematopoietic cell transplantation in mantle cell lymphoma. Best Pract Res Clin Haematol 2012;25:165-74. [PubMed]
- Amayiri N, Al-Zaben A, Ghatasheh L, et al. Hematopoietic stem cell transplantation for children with primary immunodeficiency diseases: Single center experience in Jordan. Pediatr Transplant 2013;17:394-402. [PubMed]
- Michael M, Shimoni A, Nagler A. Recent compounds for immunosuppression and experimental therapies for acute graft-versus-host disease. Isr Med Assoc J 2013;15:44-50. [PubMed]
- Sellar RS, Peggs KS. Recent progress in managing graft-versus-host disease and viral infections following allogeneic stem cell transplantation. Future Oncol 2012;8:1549-65. [PubMed]
- Harris AC, Ferrara JL, Levine JE. Advances in predicting acute GVHD. Br J Haematol 2013;160:288-302. [PubMed]
- Mengarelli A, Iori A, Guglielmi C, et al. Standard versus alternative myeloablative conditioning regimens in allogeneic hematopoietic stem cell transplantation for high-risk acute leukemia. Haematologica 2002;87:52-8. [PubMed]
- Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation 1974;18:295-304. [PubMed]
- Mizuta S, Matsuo K, Maeda T, et al. Prognostic factors influencing clinical outcome of allogeneic hematopoietic stem cell transplantation following imatinib-based therapy in BCR-ABL-positive ALL. Blood Cancer J 2012;2:e72. [PubMed]
- Bakr M, Rasheed W, Mohamed SY, et al. Allogeneic hematopoietic stem cell transplantation in adolescent and adult patients with high-risk T cell acute lymphoblastic leukemia. Biol Blood Marrow Transplant 2012;18:1897-904. [PubMed]
- Lee EJ, Han JY, Lee JW, et al. Outcome of allogeneic hematopoietic stem cell transplantation for childhood acute lymphoblastic leukemia in second complete remission: a single institution study. Korean J Pediatr 2012;55:100-6. [PubMed]
- Fagioli F, Zecca M, Rognoni C, et al. Allogeneic hematopoietic stem cell transplantation for Philadelphia-positive acute lymphoblastic leukemia in children and adolescents: a retrospective multicenter study of the Italian Association of Pediatric Hematology and Oncology (AIEOP). Biol Blood Marrow Transplant 2012;18:852-60. [PubMed]
- Ishiyama K, Takami A, Kanda Y, et al. Allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia with t(6;9)(p23;q34) dramatically improves the patient prognosis: a matched-pair analysis. Leukemia 2012;26:461-4. [PubMed]
- Döhner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood 2010;115:453-74. [PubMed]
- Estey EH. Acute myeloid leukemia: 2012 update on diagnosis, risk stratification, and management. Am J Hematol 2012;87:89-99. [PubMed]
- Kumar P, Defor TE, Brunstein C, et al. Allogeneic hematopoietic stem cell transplantation in adult acute lymphocytic leukemia: impact of donor source on survival. Biol Blood Marrow Transplant 2008;14:1394-400. [PubMed]
- Harris AC, Kitko CL, Couriel DR, et al. Extramedullary relapse of acute myeloid leukemia following allogeneic hematopoietic stem cell transplantation: incidence, risk factors and outcomes. Haematologica 2013;98:179-84. [PubMed]
- Yan CH, Liu DH, Liu KY, et al. Risk stratification-directed donor lymphocyte infusion could reduce relapse of standard-risk acute leukemia patients after allogeneic hematopoietic stem cell transplantation. Blood 2012;119:3256-62. [PubMed]


