Use of 18F-FDG PET/CT to locate primary malignancies in patients with hepatic cirrhosis and malignant ascites
Introduction
The most common cause of ascites is liver cirrhosis which accounts for more than three-quarters of all cases (1). Malignant ascites is seen in about 10% of the patients with ascites. However, it could be a diagnostic challenge for clinicians to determine whether ascites is due to benign liver cirrhosis or due to malignancies. It is especially difficult to discover malignant ascites in cirrhotic patients. The cause of ascites can be diagnosed in most cases based on history, clinical examination, serum biochemical tests, fluid biochemistry and cytology, abdominal ultrasound, and paracentesis (2,3). However, in some cases the etiology still cannot be confirmed with laboratory tests (including cell count, albumin level, total protein level, Gram staining, and culture) and imaging including ultrasound and computer tomography (CT) scan, and further investigation is necessary (4).
18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET) is emerging as a useful noninvasive imaging technique to assess biochemical and metabolic differences between benign and malignant lesions. Recently, integrated PET/CT with functional and anatomical imaging has been shown to increase the diagnostic accuracy of malignant ascites (5,6). To date, few studies have focused on the role of 18F-FDG PET/CT in the evaluation of ascites in liver cirrhosis patients. In this study, we assessed the value of 18F-FDG PET/CT in determining the cause of ascites in liver cirrhosis patients who were clinically suspected of cancer or the primary sites of the malignancy were unknown.
Materials and methods
Patients
A retrospective study of liver cirrhosis patients with ascites who were referred for 18F-FDG PET/CT because of clinical suspicion of cancer or unknown primary site was performed to determine the underlying causes of ascites and discriminate malignant ascites from benign causes.
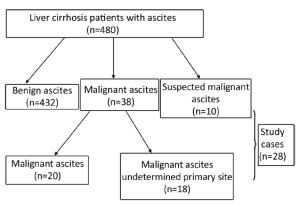
A total of 480 liver cirrhosis patients with ascites from 2008 to 2012 were screened, and those in one of the following two categories were further investigated using 18F-FDG PET/CT: (I) patients who were highly suspected of having malignancies clinically, but cytology and other noninvasive diagnostic imaging such as CT and ultrasound were negative; and (II) patients who had malignant cytological findings, but the primary sites of the cancers were not detected by CT or ultrasound. Written informed consent was obtained from all subjects.
18F-FDG PET/CT protocol
Before surgery, gastroenterological endoscope, and pleural needle biopsy, all patients underwent whole-body 18F-FDG PET imaging after 6 h fast. They had plasma glucose levels less than 140 mg/dL. 18F-FDG (370 MBq each) was injected intravenously, and emission scanning using a whole-body technique (5-12 bed positions, acquisition time 5 min/bed position) was performed 60 min after the injection. The patients then underwent a transmission scan (3 min/bed) using a rotating 68 Ge sources. The raw data were then reconstructed using ordered subset expectation maximization (OSEM) algorithms method. The PET images were evaluated blindly by two experienced physicians.
Statistical analysis
Continuous data with normal distributions were expressed as x̄±s, whereas continuous data with non-normal distributions were expressed as median (interquartile range). The Statistical Package for Social Science (SPSS), version 16.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. P<0.05 was considered statistically significant.
Results
Patient’s characteristics
Of the 480 liver cirrhosis patients with ascites enrolled in the study, 20 (4.17%) patients had a known primary malignancy diagnosed. Eighteen patients were diagnosed with cancer but of undetermined primary sites, ten were suspected of cancer, and these 28 patients were further studied using 18F-FDG PET/CT (Figure 1).
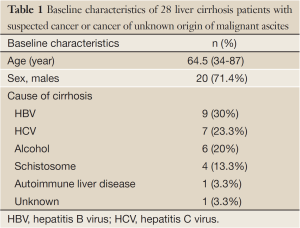
The baseline characteristics of the 28 patients including sex, age and causes of cirrhosis are summarized in Table 1. Median age was 64.5 years. Among the 28 patients, the causes of liver cirrhosis included hepatitis C virus infection (7, 25.0%), hepatitis B virus infection (9, 32.1%), autoimmune liver disease (1, 3.6%), alcoholic liver disease (6, 21.4%), schistosomiasis (4, 14.3%), and unknown (1, 3.6%).
Full Table
Diagnostic accuracy of 18F-FDG PET/CT in detection of clinically suspected cancer or cancer of unknown primary site of malignant ascites
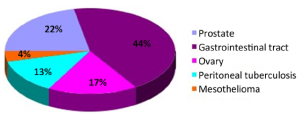
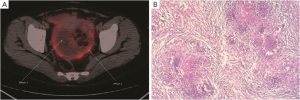
Twenty-three cases were found to have sites with increased 18F-FDG uptake including prostate 5 (21.7%), peritoneal 4 (13.3%), ovary 4 (13.3%), and gastrointestinal tract 10 (43.5%) (Figure 2). Cancers were confirmed by surgery and laparoscopic examination in 20 of the 23 patients. Three false positive patients were found to have peritoneal tuberculosis (Figures 3,4). The standardized uptake values (SUV) were significantly higher in cancers than in benign lesions (mean value, 6.95 vs. 2.94; P=0.005).
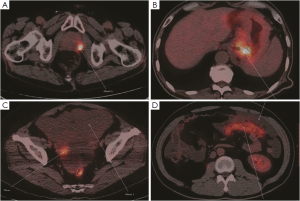
Discussion
Cancer of uncertain primary site is not an uncommon problem in clinical practice and the prognosis is usually poor (7,8). The survival depends on identifying the primary site and specific targeted therapy (9). The use of 18F-FDG PET/CT is helpful to locate the primary site of malignancy in certain groups of patients. In patients with liver cirrhosis, the appearance of ascites is usually considered to be the complication of decompensated cirrhosis. However, malignant ascites is not uncommon in these patients (1). If the diagnosis and treatment are not timely, the prognosis is extremely dismal. Therefore, liver cirrhosis patients with ascites need to be screened for malignant ascites by cytology, ascites albumin gradient, tumor markers, culture and non-invasive imaging, such as ultrasound and CT. Previous studies have shown that PET/CT has high diagnostic accuracy in mesothelioma, digestive tract cancer, prostate cancer and ovarian cancer, especially when the primary site of the tumor is uncertain (10-16). A more individualized treatment strategy is dependent on accurate pathological diagnosis. PET/CT guided fine needle biopsy can obtain tissue from high 18F-FDG uptake area avoiding false positive results. In this study, we found that in patients with liver cirrhosis who are clinically suspected of cancer or have cancer of unknown primary sites, 18F-FDG PET/CT can significantly reduce diagnostic uncertainty.
The potential ability of 18F-FDG PET/CT to determine the tissue origin of cancer could be a very useful tool for more specific, less toxic treatment of cancer. Localization of the tumor by 18F-FDG PET/CT plays an important role in radiation therapy to avoid damage to the normal tissues (17). Liver cirrhosis patients with malignant ascites sometimes cannot tolerate surgical treatment due to poor general condition. The appearance of malignant ascites in liver cirrhosis patients often indicates metastasis of tumor. Radiotherapy becomes the only choice and PET/CT also has its unique advantage in guiding radiotherapy (18) in terms of the radiation site, and dose distribution. For radiotherapy, CT is the standard method for tumor volume delineation. The major shortcoming is its inability to detect residual tumor and recurrence. PET-based treatment planning in radiotherapy can adjust the radiation field according to the edge of the tumor, increase the radiation dose to the target area, and at the same time, avoid the normal tissue. Adjustment of radiotherapy can also be made according to the patient’s response (19). Ten patients in this study received radiotherapy without adverse reactions, but the long-term prognosis still needs further follow-up.
This study has several limitations. The retrospective study has case selection bias affecting the representativeness and credibility. In addition, the case number of this study is relatively small. There were also three false positive results (tuberculosis). It’s been documented that FDG uptake in tuberculosis lesions is sparse (20). However, our study suggests that the patterns of FDG accumulation in tuberculosis need to be further evaluated (21).
In conclusion, 18F-FDG PET/CT can be as a powerful imaging tool in identifying malignant origin in liver cirrhosis patients who are clinically suspected of cancers or have cancers of unknown primary sites. We believe that 18F-FDG PET/CT is a valuable alternative to current diagnostic methods for identifying uncertain primary cancers. Further studies for evaluating the value of 18F-FDG PET/CT on treatment choice and outcome for those patients are warranted.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Reynolds TB. Ascites. Clin Liver Dis 2000;4:151-68. [PubMed]
- European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol 2010;53:397-417. [PubMed]
- Moore KP, Wong F, Gines P, et al. The management of ascites in cirrhosis: report on the consensus conference of the International Ascites Club. Hepatology 2003;38:258-66. [PubMed]
- Han CM, Lee CL, Huang KG, et al. Diagnostic laparoscopy in ascites of unknown origin: Chang Gung Memorial Hospital 20-year experience. Chang Gung Med J 2008;31:378-83. [PubMed]
- Li XJ, Li FQ, Han JK, et al. Ascites metabolism measurement enhanced the diagnostic value and accuracy of prognostic evaluation in 18F-FDG PET/CT studies in malignant ascites patients. Nucl Med Commun 2013;34:544-50. [PubMed]
- Townsend DW. Positron emission tomography/computed tomography. Semin Nucl Med 2008;38:152-66. [PubMed]
- Monzon FA, Medeiros F, Lyons-Weiler M, et al. Identification of tissue of origin in carcinoma of unknown primary with a microarray-based gene expression test. Diagn Pathol 2010;5:3. [PubMed]
- Pavlidis N, Fizazi K. Carcinoma of unknown primary (CUP). Crit Rev Oncol Hematol 2009;69:271-8. [PubMed]
- Varadhachary G. New Strategies for Carcinoma of Unknown Primary: the role of tissue of origin molecular profiling. Clin Cancer Res 2013;19:4027-33. [PubMed]
- Fuccio C, Spinapolice EG, Ferretti A, et al. 18F-FDG-PET/CT in malignant mesothelioma. Biomed Pharmacother 2013;67:539-42. [PubMed]
- Hinev A, Chaushev B, Klisarova A. FDG PET/CT in Prostate Cancer: A valuable method to detect the primary and metastatic tumor sites and to monitor cancer response to hormonal therapy. Nephrourol Mon 2012;4:644-5. [PubMed]
- Zhang M, Jiang X, Zhang M, et al. The Role of 18F-FDG PET/CT in the evaluation of Ascites of Undetermined Origin. J Nucl Med 2009;50:506-12. [PubMed]
- Karapolat I, Kumanlıoğlu K. Impact of FDG-PET/CT for the detection of unknown primary tumours in patients with cervical lymph node metastases. Mol Imaging Radionucl Ther 2012;21:63-8. [PubMed]
- Wang G, Wu Y, Zhang W, et al. Clinical value of whole-body F-18 fluorodeoxyglucose positron emission tomography/computed tomography in patients with carcinoma of unknown primary. J Med Imaging Radiat Oncol 2013;57:65-71. [PubMed]
- Ozpacaci T, Tamam MO, Mulazimoglu M, et al. Isolated adrenal metastasis of small cell neuroendocrine carcinoma of the ovary detected with FDG-PET/CT. Rev Esp Med Nucl Imagen Mol 2012;31:297-8. [PubMed]
- Fahim-Ul-Hassan, Cook GJ. PET/CT in oncology. Clin Med 2012;12:368-72. [PubMed]
- Li J, Xiao Y. Application of FDG-PET/CT in Radiation Oncology. Front Oncol 2013;3:80. [PubMed]
- Facciuto ME, Singh MK, Rochon C, et al. Stereotactic body radiation therapy in hepatocellular carcinoma and cirrhosis: evaluation of radiological and pathological response. J Surg Oncol 2012;105:692-8. [PubMed]
- Tsurusaki M, Okada M, Kuroda H, et al. Clinical application of 18F-fluorodeoxyglucose positron emission tomography for assessment and evaluation after therapy for malignant hepatic tumor. J Gastroenterol 2013. [Epub ahead of print]. [PubMed]
- Bakheet SM, Powe J, Ezzat A, et al. F-18-FDG uptake in tuberculosis. Clin Nucl Med 1998;23:739-42. [PubMed]
- Tsujimoto N, Saraya T, Takizawa H, et al. Tuberculous peritonitis incidentally diagnosed on FDG-PET/CT. Intern Med 2013;52:841-2. [PubMed]