Imatinib mesylate in clinically suspected gastric stromal tumors
Introduction
Gastrointestinal stromal tumors (GISTs) are uncommon mesenchymal tumors that arise in the wall of the gastro-intestinal (GI) tract and are less than 3% of all GI malignancies (1). GISTs occur most frequently in the stomach (60% to 70%), followed by the small intestine (20% to 30%), colon and rectum (5%), and esophagus (less than 5%). GIST is different from cancer in that its growth is mostly expansive rather than invasive growth and its main metastatic routes are hematogenous and seeding metastasis (2). GISTs should be handled carefully to avoid bleeding or tumor spillage. Diagnosis of gastric GIST is not always clear before surgery. Flexible endoscopy may suggest the nature of the lesion (a bulky tumor with preserved mucosa); however, biopsy is rarely diagnostic. Although recent advances in imaging techniques, such as ultrasonography, computed tomography, and magnetic resonance imaging (MRI), have aided in the identification of space-occupying lesions of the stomach, these techniques do not permit preoperative definitive diagnosis. Furthermore, symptoms change depending on size, location, and growth (endoluminal or extraluminal) of the tumor. Therefore, diagnostic medication with safe drugs may provide a feasible way under such conditions after an informed consent is obtained. Based on the excellent efficacy of imatinib mesylate (IM) in the treatment of GIST, we applied it in the diagnostic medication of clinically suspected gastric stromal tumors.
Case study
Case 1
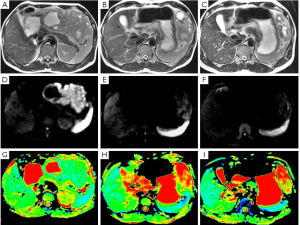
A 44-year-old male patient was admitted. Six months ago, a space-occupying lesion was found in his stomach in a CT scan performed in a local hospital, and was suspected to be GIST. No tumor tissue was obtained upon endoscopic biopsy, and he refused needle biopsy. The local hospital suggested surgical treatment, and meanwhile informed him that there was a possibility of concomitant splenectomy. The patient refused the surgery. He was then referred to our hospital. GIST was considered after MRI. Diagnostic medication with IM (for one month) was effective (Figure 1). Six months later, the treatment was assessed as partially responsive (PR), with tumor size decreased from 11.7 to 5.5 cm. No other abnormality was detected upon admission. He received surgical treatment under general anesthesia in March 2009. Intraoperative exploration showed that the mass was located near the greater curvature of the stomach, and no obvious adhesion with spleen and colon was observed. Partial gastrectomy was performed using linear cutter. Pathology prompted a GIST sized 9 cm × 9 cm × 4 cm. The tumor tissue is associated with hyaline degeneration and invades the submucosa. The cutting edge of the tumor is negative. The omental tissue showed no abnormality. After the surgery, he received medical treatment for up to one year. Till now his disease-free survival has reached 39 months.
Case 2
A 45-year-old male patient was admitted. During his health-check in a local hospital, abdominal ultrasound showed “space-occupying lesion in the left upper abdomen”. In our center, CT showed an irregular soft tissue mass (sized 91 mm × 70 mm) in the left upper abdomen; contrast-enhanced scanning showed non-homogeneous enhancement. The boundary between the mass and the posterior wall of the stomach and the tail of the pancreas is unclear. GIST is considered. No tumor tissue was obtained upon endoscopic biopsy, and he refused needle biopsy. MRI prompted GIST. Diagnostic medication with IM (for five weeks) was effective and lasted for three months. MRI re-examination showed similar results. The patient requested surgical treatment. He then underwent partial gastrectomy in March 2012. Intra-operative exploration showed that the mass was originated from the fundic posterior wall that is near the greater curvature of the stomach and grew outside gastric wall, without obvious involvement with the surrounding organs. The mass was soft, dark red in color, and sized about 8 cm. After the adhesion is separated, dissect the mass from the posterior wall of the stomach, with about 4 cm of the gastric wall was involved. Pathology showed that there was a 9.5 cm × 8 cm × 6.5 cm mass (most inside the abdominal cavity and part in the stomach), with smooth surface. The mass was attached with a few mucous tissue sized 2.5 cm × 1.5 cm. The tumor section was grey-yellow or grey-red and solid, with some hemorrhage and necrosis. The tumor invaded the muscular layer of the gastric wall and the subserosal tissue. Mitotic figures were <1/50 at high magnification. Immunohistochemical examinations showed tumor cells CD117 (+), CD34 (+), Desmin (-), DOG1 (+), Ki-67 (+<1%), S-100 (-), and SMA (-), which supported the above diagnosis. Currently the patient is still on IM treatment.
Discussion
GIST is the most common mesenchymal tumor in the gastrointestinal tract. GIST is not responsive to routine chemotherapy, and surgical treatment remains the only option. However, surgery alone is not sufficient. IM-based molecular targeted therapy has showed promising efficacy for the relapsed or metastatic GIST. Meanwhile, IM has also played a key role in the neoadjuvant therapy of GIST (3). Along with the increased knowledge on GIST, improved drug efficacy, and more sophisticated imaging techniques, the imaging-based diagnosis and efficacy evaluation have rapidly advanced, and new efficacy criteria have been developed (4). In our clinical practice, quite a few GIST patients developed structural changes on imaging examinations after several weeks (and even several days) of IM treatment (5). Thus, for patients whose pathological examination results can not be obtained due to the above mentions reasons, a diagnostic medication with IM may be considered: the drug is effective and safe, and its efficacy can be accurately reflected upon imaging examinations. The clinical application of diagnostic medication is often limited by concerns including the effectiveness and side effects of the drug and the accuracy of the diagnosis; fortunately, IM makes these concerns unnecessary due to its extraordinary advantages. Our cases provided useful experiences in the diagnostic medication of IM, within a time frame that is accepted by both physicians and patients, IM can safely and reliably achieve structural changes that can be identified by imaging examinations. If the medication is not effective, the diagnosis of GIST then can not be confirmed. In the practice, a main problem pertaining to short-term IM usage is its cost. It is therefore recommended that the diagnostic medication with IM can be an alternative option for patients with suspected GIST that can not be confirmed pathologically.
Acknowledgements
This study was supported by WU JIEPING Medical Foundation (No. WJP-320.6700.09010).
Disclosure: The authors declare no conflict of interest.
References
- Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol 2002;33:459-65. [PubMed]
- Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 2002;347:472-80. [PubMed]
- Eisenberg BL, Harris J, Blanke CD, et al. Phase II trial of neoadjuvant/adjuvant imatinib mesylate (IM) for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumor (GIST): early results of RTOG 0132/ACRIN 6665. J Surg Oncol 2009;99:42-7. [PubMed]
- Choi H, Charnsangavej C, Faria SC, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol 2007;25:1753-9. [PubMed]
- Tang L, Zhang XP, Sun YS, et al. Gastrointestinal stromal tumors treated with imatinib mesylate: apparent diffusion coefficient in the evaluation of therapy response in patients. Radiology 2011;258:729-38. [PubMed]


