Can statins reduce risk of lung cancer, especially among elderly people? A meta-analysis
Introduction
Statins are commonly used as a kind of cholesterol-lowering agents (1,2). They were reported to suppress tumor cell growth in several in vitro studies (3,4) and animal experiments (5,6). Recently, statins have become the most popular drugs used for high cholesterol because of their efficacy and few side effects. Besides, some studies have shown that statins have antiproliferative, proapoptotic and antiinvasive effects (7,8). Thus, many scholars show increasing interest in the antitumor effect of statins.
Among so many cancers, lung cancer is the most common cause of cancer mortality throughout the world with poor prognosis (9). In 2008, there were 1.61 million new cases, and 1.38 million deaths due to lung cancer, especially in Europe and North America (10). Common treatments include palliative care, surgery, chemotherapy, and radiation therapy. So people have tried to find out effective ways in preventing lung cancer.
Some meta-analyses (11-14) have yielded inconsistent results on chemopreventive effect of statins on lung cancers. In vitro data have supported a potential role for statins in preventing cancer risk. HMG-CoA reductase overexpressed in cancer cells (15), and statins have been found to induce apoptosis in cancer cell lines (16,17). In contrast, Newman and Hulley (18) reported that lipid-lowering therapy might cause cancers in rodents. So there is no final conclusion about the anticancer effect of statins. The aim of this meta-analysis was to evaluate the protective association between statins and lung cancer risk, especially among elderly people.
Materials and methods
Search strategy
We conducted a computer-assisted systematic search of MEDLINE, EMBASE, and Web of Science databases from their commencement to September, 2013, attempting to find all publications on the effect of statins on lung cancer. Key words and medical subject heading (MeSH) terms for the search of MEDLINE were as follows: [“Lung cancer” (MeSH)] AND [“statins” (MeSH) OR “HMG-CoA-reducatase-inhibitor” OR pravastatin OR simvastatin OR lovastatin OR atorvastatin OR cerivastatin OR rosuvastatin OR fluvastatin]. We used similar strategies to search EMBASE. Web of Science was searched mainly for the abstracts of additional oncology society meetings. We also reviewed the bibliographies of relevant articles to identify additional studies that might have been missed.
Selection criteria
We screened titles and abstracts of identified papers to exclude studies that clearly did not meet the inclusion criteria. Full texts of those selected studies for further review were retrieved and evaluated. The studies included in this meta-analysis evaluated the statin use on lung cancer risk, but the statin use on the lung cancer mortality was excluded. The studies must test on human except specific population (e.g., patients after heart transplantation) (19), and in vitro experiments and animal trials were not included. To make sure the comparability of all the included studies, we made some other criteria to study selection and data extraction. Inclusion criteria were: publications in English; full texts can be found; an original study comparing statins group with placebo group or no statins group; follow up for over one year; lung cancer must be included in the cancer outcomes; and the strength of the association between statins and lung cancer must have measured in the form of odds ratio (OR), risk ratio (RR) or hazard ratio (HR), or could be calculated from the original data presented in the studies.
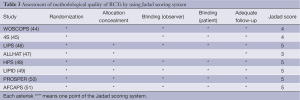
We evaluated the methodological quality of all randomized controlled trials (RCTs) by using Jadad scoring system (20). Studies would be regarded as good methodological quality with scores not less than three points. Besides, we used a subgroup analysis to evaluate some influencing factors for the effect of statins on lung cancer risk (21,22).
Data extraction
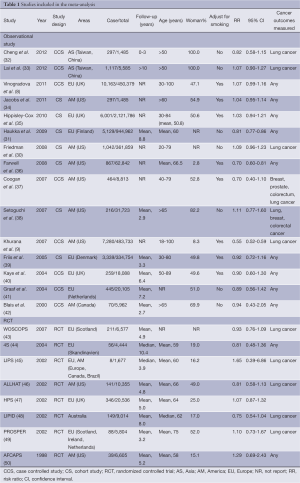
All data were extracted according to the criteria. Discrepancies were discussed and resolved by consensus. Data extracted from each study included the first author, year of publication, regions of the population investigated, lung cancer cases/No. of all the participants, follow-up, age, the percentage of women, cancer outcomes, adjustment for smoking, adjusted OR/RR/HR, and 95% confidence interval (95% CI).
STATA Statistical Software was used for all the analyses (version 12.0, STATA Corporation, College Station, TX, USA). The measure of estimated effect of interest was RR with 95% CI. Because the risk of lung cancer is relatively low, the RR mathematically approximates the OR, or HR in case-control and cohort studies. We therefore reported all results as the RR for simplicity. Summary RR estimates were calculated using RR, OR, or HR reported in each study.
Meta-analysis
We used two models to calculate the pooled RR estimates with 95% CI: a fixed-effects model known as Mantel-Haenszel method (23) and a random-effects model known as DerSimonian-Laird method (24). When RR <1 and upper limit of 95% CI was lower than 1, we considered statin use could reduce the risk of lung cancer with statistical significance. We used the Cochran Q test to evaluate the heterogeneity of the studies (25) and the quantity I2 was also calculated (26,27). I2 is the proportion of total variation contributed by between-study variation, and values of 25%, 50%, and 75% have been regarded as representing low, moderate, and high heterogeneity respectively.
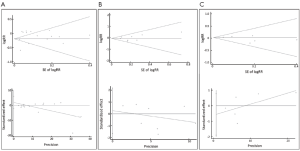
Publication bias was evaluated to find whether the results of the studies were homogeneous. The funnel graph, the Egger regression asymmetry test (28) and the Begg-Mazumdar adjusted rank correlation test (29) were used. When the P value of the Egger’s test and Begg’s test was <0.05, we considered obvious bias among the studies.
Subgroup analysis
We performed subgroup analysis according to: (I) study design: case-control study and cohort study; (II) regions of the investigated population: Asia, America and Europe; (III) the percentage of women: the group ≥50% and the group <50%; (IV) the mean follow-up of the studies: group ≥5 years and the group <5 years (we chose 5 years as the boundary because in some studies (30,31), long-term effect of statin therapy was considered for more than 5 years); and (V) adjustment for smoking, because the risk of lung cancer is obviously associated with smoking.
Results
Search results
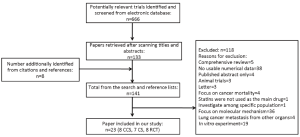
We found 561 records in EMBASE Database, 69 records in Web of Science Database and 36 records in MEDLINE Database. Eight references were found from the reference lists. With our selection criteria, we identified 23 studies to our meta-analysis, including 15 observational studies (8,9,30-42) (8 case-control studies, 7 cohort studies) and 8 randomized-controlled studies (43-50) (Figure 1). Table 1 summarizes the characteristics of all the included studies.
Full table
Meta-analysis of statin use and risk of lung cancer
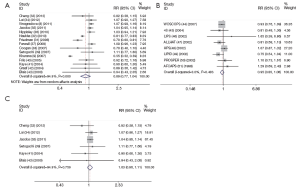
The meta-analysis of 15 observational studies showed no evidence for a protective association between statin use and the risk of lung cancer with obvious heterogeneity (RR: 0.89, 95% CI: 0.77-1.04, I2=94.9%, P=0) (Figure 2, Table 2). Begg’s test (P=0.235) and Egger’s test (P=0.356) showed no obvious publication bias (Figure 3, Table 2). The assessment of methodological quality of RCTs by using Jadad scoring system was illustrated in Table 3, and 5 RCTs scored 5, 2 RCTs scored 4 and 1 RCT scored 3. All the RCTs were regarded as studies with good methodological quality. And the meta-analysis of 8 RCTs also showed no association between statins use and lung cancer risk (RR: 0.95, 95% CI: 0.85-1.06, I2=0, P=0.483) (Figure 2, Table 2). No obvious publication bias was found (Begg’s test: P=0.536; Egger’s test: P=0.743) (Figure 3, Table 2).

Full table
Full table
Because the heterogeneity of all the observational studies was very obvious and obvious heterogeneity made the results less credible, we used an age limitation among all the 15 observational studies. And then we selected six observational studies that all the participants were elderly people (age >50 years old) (32-34,38,40,42), meta-analysis of this 6 studies still showed no protective effect on lung cancer among elderly people with no heterogeneity (RR: 1.03, 95% CI: 0.96-1.11, I2=0, P=0.759) (Figure 2, Table 2). And no obvious publication bias was found (Begg’s test: P=0.707; Egger’s test: P=0.312) (Figure 3, Table 2). So we could draw the conclusion that there was no protective effect between statin use and lung cancer risk among elderly people.
Subgroup analysis of statin use and lung cancer risk
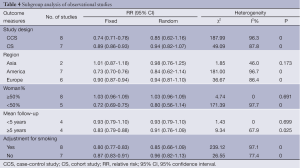
We performed a subgroup analysis according to study design, investigation regions, mean follow-up, gender and adjustment for smoking. Table 4 summarizes the results of the subgroup analysis on 15 observational studies.
Full table
In the subgroup of study design, both case-control studies and cohort studies showed that statins had no association with the risk of lung cancer (Table 4).
In the subgroup of investigation regions, studies in Asia, Europe and America all showed no protective effect of statin use on lung cancer risk (Table 4). The results pointed out that the effect of statins has no association with areas.
We also took into account the effect of gender on the results. In the subgroup of the percentage of women, the studies that the number of women was more than 50% and less than 50% both showed there was no association between statins and lung cancer risk (Table 4). So gender was not an influence factor of the statin effect.
In the subgroup of the mean follow-up years, the group <5 years and the group ≥5 years showed no protective association between statins and cancer risk (Table 4). It hinted that long-term statin use also had no protective effect on lung cancer risk.
Because the risk lung cancer was strongly associated with smoking, the adjustment for smoking might affect the results of the researches. But in our subgroup analysis, the group with adjustment for smoking and the group without adjustment for smoking both showed no association between statin use and lung cancer risk (Table 4).
Discussion
Although our meta-analysis showed that statins had inconsistent effect on the overall lung cancer risk and elderly people’s lung cancer risk, it could be affected by many factors. Many factors were not considered in our meta-analysis and the previous meta-analysis, such as doses, races, body mass index (BMI), and other lung diseases like chronic obstructive pulmonary disease (COPD).
Four meta-analyses of Bonovas et al. (11), Dale et al. (14), Kuoppala et al. (13) and Browning et al. (12) had investigated the association between statin therapy and cancer risk, and all showed that there was no evidence to prove the association between statin therapy and cancer risk. But they all focused on the overall cancer risk. Our study emphasized the statin effects on the risk of lung cancer, especially among elderly people. Although our results also pointed out there is no evidence to prove the association between statin use and lung cancer risk, it was further showed that statin use had no protective effect on lung cancer among elderly people. This result was different from the studies of Khurana et al. (9) and Farwell et al. (36). Khurana et al. showed statin use could reduce lung cancer risk (RR: 0.55, 95% CI: 0.52-0.59) and Farwell et al. showed the reduction of lung cancer risk (RR: 0.70, 95% CI: 0.60 to 0.81) on veterans. But few RCTs focused on the effect of statins on lung cancer risk among elderly people. All these results will raise the attention to the prevention of lung cancer by using statins among elderly people.
On the other hand, our study pointed out long-term effect of statins (≥5 years) has no protective effect on lung cancer risk, but the long-term effects of statins are always contentious. Study by Setoguchi et al. (38) showed long-term users of statins had no protective effect of lung cancer risk (RR: 1.11, 95% CI: 0.77-1.60), which was similar to the study by Gary D. Friedman et al. (30) showing long-term statin use did not reduce the risk of lung cancer (RR: 1.09, 95% CI: 0.96-1.23). And Vinogradova et al. (8) even showed that long-term use of statins would increase the risk of lung cancer (RR: 1.18, 95% CI: 1.05-1.34). Although statins have few side effects, the safety of long-term effect of statins should be paid attention.
Our study has several strengths. Firstly, our study included observational studies (cohort studies and case-control studies) and RCTs. This has allowed us to perform the analysis separately by difference study designs and make the results more convincing. Secondly, we selected six observational studies which focused on elderly people to show the effect of statins. Thirdly, all pooled RR estimates were calculated by the fixed-effects model and the random-effects model. The publication bias was calculated by the Begg’s test and the Egger’s test to make sure any bias from misclassification is likely to be small. Fourthly, subgroup analysis was performed to analyze the influencing factors of statin therapy on lung cancer risk.
But our study also has some limitations. Firstly, information on risk factors for lung cancer, such as level of physical activity, obesity, alcohol use, smoking status and diets, were not included in the study. Secondly, the information on statin therapy on patients was also not classified by the types of statins, and the dose of daily use. Thirdly, the meta-analysis among elderly people is based on observational studies, which needs more RCTs and further studies on it. Fourthly, we used the adjusted RR to contribute to our meta-analysis, but every study adjusted their results by difference factors.
To date, no effective chemopreventive agent is identified for lung cancer. A study by Nowak et al. (51) suggested that high levels of beta-carotene in the diet or in the blood were associated with low risk of lung cancer, while a study by Boone et al. (52) and a study by Woodson et al. (53) suggested that low levels of vitamins A and E in blood were associate with the development of lung cancer. And then, the study by Newman et al. (18) on animal models showed that statins might cause cancer in rodents. More and more studies on statin use against lung cancer are in progress, and we look forward to further studies on statin use against lung cancer.
Acknowledgements
This study was supported by Key Laboratory of Medical Molecular Technology, Medical School of Nanjing University and The Tumor Hospital of Jiangsu Province.
Disclosure: The authors declare no conflict of interest.
References
- Siegel D, Lopez J, Meier J. Use of cholesterol-lowering medications in the United States from 1991 to 1997. Am J Med 2000;108:496-9. [PubMed]
- Lemaitre RN, Furberg CD, Newman AB, et al. Time trends in the use of cholesterol-lowering agents in older adults-The Cardiovascular Health Study. Arch Intern Med 1998;158:1761-8. [PubMed]
- Carlberg M, Dricu A, Blegen H, et al. Mevalonic acid is limiting for N-linked glycosylation and translocation of the insulin-like growth factor-1 receptor to the cell surface. Evidence for a new link between 3-hydroxy-3-methylglutaryl -coenzyme a reductase and cell growth. J Biol Chem 1996;271:17453-62. [PubMed]
- Addeo R, Altucci L, Battista T, et al. Stimulation of human breast cancer MCF-7 cells with estrogen prevents cell cycle arrest by HMG-CoA reductase inhibitors. Biochem Biophys Res Commun 1996;220:864-70. [PubMed]
- Lubet RA, Boring D, Steele VE, et al. Lack of Efficacy of the statins atorvastatin and lovastatin in rodent mammary carcinogenesis. Cancer Prev Res (Phila) 2009;2:161-7. [PubMed]
- Inano H, Suzuki K, Onoda M, et al. Anti-carcinogenic activity of simvastatin during the promotion phase of radiation-induced mammary et altumorigenesis of rats. Carcinogenesis 1997;18:1723-7. [PubMed]
- Chan KK, Oza AM, Siu LL. The statins as anticancer agents. Clin Cancer Res 2003;9:10-9. [PubMed]
- Vinogradova Y, Coupland C, Hippisley-Cox J. Exposure to statins and risk of common cancers: a series of nested case-control studies. BMC cancer 2011;11:409. [PubMed]
- Khurana V, Bejjanki HR, Caldito G, et al. Statins reduce the risk of lung cancer in humans: a large case-control study of US veterans. Chest 2007;131:1282-8. [PubMed]
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893-917. [PubMed]
- Bonovas S, Filioussi K, Tsavaris N, et al. Statins and cancer risk: a literature -based meta-analysis and meta-regression analysis of 35 randomized controlled trials. J Clin Oncol 2006;24:4808-17. [PubMed]
- Browning DR, Martin RM. Statins and risk of cancer: a systematic review and metaanalysis. Int J Cancer 2007;120:833-43. [PubMed]
- Kuoppala J, Lamminpaa A, Pukkala E. Statins and cancer: A systematic review and meta-analysis. Eur J Cancer 2008;44:2122-32. [PubMed]
- Dale KM, Coleman CI, Henyan NN, et al. Statins and cancer risk-a meta-analysis. JAMA 2006;295:74-80. [PubMed]
- Hentosh P, Yuh SH, Elson CE, et al. Sterol-independent regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase in tumor cells. Mol Carcinog 2001;32:154-66. [PubMed]
- Dimitroulakos J, Nohynek D, Backway KL, et al. Increased sensitivity of acute myeloid leukemias to lovastatin-induced apoptosis: A potential therapeutic approach. Blood 1999;93:1308-18. [PubMed]
- Rao CV, Newmark HL, Reddy BS. Chemopreventive effect of farnesol and lanosterol on colon carcinogenesis. Cancer Detect Prev 2002;26:419-25. [PubMed]
- Newman TB, Hulley SB. Carcinogenicity of lipid-lowering drugs. JAMA 1996;275:55-60. [PubMed]
- Fröhlich GM, Rufibach K, Enseleit F, et al. Statins and the risk of cancer after heart transplantation. Circulation 2012;126:440-7. [PubMed]
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1-12. [PubMed]
- Hasselblad V, Eddy DM, Kotchmar DJ. Synthesis of environmental evidence -nitrogen-dioxide epidemiology studies. J Air Waste Manage Assoc 1992;42:662-71. [PubMed]
- Friedenreich CM, Brant RF, Riboli E. Influence of methodologic factors in a pooled analysis of 13 case-control studies of colorectal-cancer and dietary fiber. Epidemiology 1994;5:66-79. [PubMed]
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959;22:719-48. [PubMed]
- DerSimonian R, Laird N. Metaanalysis in clinical-trials. Control Clin Trials 1986;7:177-88. [PubMed]
- Cochran WG. The combination of estimates from different experiments. Biometrics 1954;10:101-29.
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58. [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [PubMed]
- Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [PubMed]
- Begg CB, Mazumdar M. Operating characteristics of a bank correlation test for publication bias. Biometrics 1994;50:1088-101. [PubMed]
- Friedman GD, Flick ED, Udaltsova N, et al. Screening statins for possible carcinogenic risk: up to 9 years of follow-up of 361,859 recipients. Pharmacoepidemiol Drug Saf 2008;17:27-36. [PubMed]
- Haukka J, Sankila R, Klaukka T, et al. Incidence of cancer and statin usage -record linkage study. Int J Cancer 2010;126:279-84. [PubMed]
- Cheng MH, Chiu HF, Ho SC, et al. Statin use and the risk of female lung cancer: a population-based case-control study. Lung Cancer 2012;75:275-9. [PubMed]
- Lai SW, Liao KF, Lin CL, et al. Statins use and female lung cancer risk in Taiwan. Libyan J Med 2012;7:1-3. [PubMed]
- Jacobs EJ, Newton CC, Thun MJ, et al. Long-term use of cholesterol-lowering drugs and cancer incidence in a large United States cohort. Cancer Res 2011;71:1763-71. [PubMed]
- Hippisley-Cox J, Coupland C. Unintended effects of statins in men and women in England and Wales: population based cohort study using the QResearch database. BMJ 2010;340:c2197. [PubMed]
- Farwell WR, Scranton RE, Lawler EV, et al. The association between statins and cancer incidence in a veterans population. J Natl Cancer Inst 2008;100:134-9. [PubMed]
- Coogan PF, Rosenberg L, Strom BL. Statin use and the risk of 10 cancers. Epidemiology 2007;18:213-9. [PubMed]
- Setoguchi S, Glynn RJ, Avorn J, et al. Statins and the risk of lung, breast, and colorectal cancer in the elderly. Circulation 2007;115:27-33. [PubMed]
- Friis S, Poulsen AH, Johnsen SP, et al. Cancer risk among statin users: a population-based cohort study. Int J cancer 2005;114:643-7. [PubMed]
- Kaye JA, Jick H. Statin use and cancer risk in the General Practice Research Database. Br J Cancer 2004;90:635-7. [PubMed]
- Graaf MR, Beiderbeck AB, Egberts AC, et al. The risk of cancer in users of statins. J Clin Oncol 2004;22:2388-94. [PubMed]
- Blais L, Desgagne A, LeLorier J. 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors and the risk of cancer: A nested case-control study. Arch Intern Med 2000;160:2363-8. [PubMed]
- Ford I, Murray H, Packard CJ, et al. Long-term follow-up of the west of scotland coronary prevention study. N Engl J Med 2007;357:1477-86. [PubMed]
- Strandberg TE, Pyorala K, Cook TJ, et al. Mortality and incidence of cancer during 10-year follow-up of the Scandinavian Simvastatin Survival Study (4S). Lancet 2004;364:771-7. [PubMed]
- Serruys PW, de Feyter P, Macaya C, et al. Fluvastatin for prevention of cardiac events following successful first percutaneous coronary intervention: a randomized controlled trial. JAMA 2002;287:3215-22. [PubMed]
- ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. the Antihypertensie and Lipid-Lowering Treatent to Prevent Heart Attack Trial. Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: the Antihypertensive and Lipid -Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT). JAMA 2002;288:2998-3007. [PubMed]
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002;360:7-22. [PubMed]
- LIPD Study Group (Long-term Intervention with Pravastatin in Ischaemic Disease. Long-term effectiveness and safety of pravastatin in 9014 patients with coronary heart disease and average cholesterol concentrations: the LIPID trial follow-up. Lancet 2002;359:1379-87. [PubMed]
- Shepherd J, Blauw GJ, Murphy MB, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 2002;360:1623-30. [PubMed]
- Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: Results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention /study. JAMA 1998;279:1615-22. [PubMed]
- Nowak R. Cancer Preventio. Beta-carotene - helpful or harmful. Science 1994;264:500-1. [PubMed]
- Boone CW, Kelloff GJ, Malone WE. Identification of candidate cancer chemopreventive agents and their evaluation in animal-models and human clinical-trials - a review. Cancer Res 1990;50:2-9. [PubMed]
- Woodson K, Tangrea JA, Barrett MJ, et al. Serum alpha-tocopherol and subsequent risk of lung cancer among male smokers. J Nat Cancer Inst 1999;91:1738-43. [PubMed]


