Risk factors of CMV infection in patients after umbilical cord blood transplantation: a multicenter study in China
Introduction
Human cytomegalovirus (CMV) infection is a major problem after hematopoietic stem cell transplantation (HSCT), and is associated with considerable morbidity and mortality (1). Similar to human immunodeficiency virus (HIV) infection vs. acquired immunodeficiency syndrome (AIDS), CMV infection (isolation of the CMV virus or detection of viral components in body fluid or tissue specimen) does not necessarily means the full-blown CMV disease. The current approach to monitor CMV infection is using polymerase chain reaction (PCR) to detect plasma CMV pp65 antigen or CMV DNA (2).
Umbilical cord blood transplantation (UCBT) has been increasingly used in patients with a variety of hematological and non-hematological diseases (3-5). In comparison to bone marrow transplantation (BMT), UCBT offers the advantages of easy procurement and less risk to donors. The risk of transmitting infectious agents and developing graft-versus-host disease (GVHD) is also lower without affecting the efficacy (6,7). A major disadvantage of UCBT is delayed immune reconstitution, and thus increased risk for infections, particularly CMV infection (8,9). Despite of the high rate of CMV infection after UCBT (vs. HSCT), the long-term survival does not seem to be affected (10).
Several studies focused on CMV infection following UCBT (11-15). These studies are limited by relatively small sample size [140 patients in the largest study (11)] and short follow-up period. As a result, the risk factors for CMV infection after UCBT remain poorly defined. The current study included 204 patients receiving UCBT, with a median follow-up period of >2 years.
Materials and methods
Patients
The study included 204 consecutive patients receiving UCBT from unrelated donors for hematological malignancies during a period from April 2000 and March 2013 at Anhui Provincial Hospital (an affiliate of Anhui Medical University), Shandong Second Hospital (an affiliate of Shandong University School of Medicine), or Wuhu Yijishan Hospital (an affiliate of Wannan Medical College). The subjects opted for UCBT since no human leukocyte antigen (HLA)-compatible related donors or suitable unrelated bone marrow donors were identified. CMV testing showed negative results prior to the transplantation in all cases. The judgment criteria of high-risk, refractory and disease progression are consistent with the reported literature (16). The study was approved by the Institutional Review Boards of the respective institutions. Written informed consent for the treatment was obtained from the patients or guardians. The study was performed in compliance with the Helsinki Declaration.
Out of the 204 cases, 28 patients deceased prior to engraftment, and did not include in data analysis. The final data analysis included 176 cases. The demographics and clinical characteristics of the subjects are shown in Table 1.
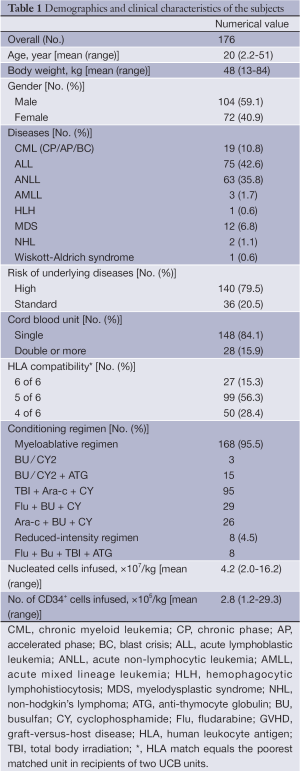
Full table
UCB unit selection and management
UCB units were identified by and obtained from the Chinese Cord Blood Bank Network. All units (as well as the mothers) were negative for immunoglobulin M antibody to CMV. All UCB units matched with the receiver at four or more of the six HLA loci. The HLA-A and HLA-B antigens were typed using standard serological techniques. HLA-DRB1 alleles were typed using high-resolution DNA techniques. The minimum cryopreserved dose for a single unit was >3×107 nucleated cells/kg and 1.2×105 CD34+cells/kg. If the minimum cell dose could not be reached with a single UCB unit, the patient received mixed units at a minimum dose of 3.5×107 nucleated cells/kg and 2×105 CD34+cells/kg. In addition to CMV, all UCB units were negative for HIV, hepatitis B virus (HBV), hepatitis C virus (HCV), as well as type I human T cell lymphotropic virus. All UCB units were transfused via a central venous route.
Transplantation procedures
HLA matching, conditioning regimen, GVHD prophylaxis, and UCB graft cell dose are shown in Table 1. Out of the 176 cases included in data analysis, 168 patients (95.5%) were treated with myeloablative conditioning regimen; the remaining 8 patients received reduced-intensity regimen. The myeloablative regimen was based on total body irradiation (TBI) or systemic chemotherapy, with or without anti-thymocyte globulin (ATG). All patients received cyclosporine A (CSA) (Novartis, Stein, Switzerland) and mycophenolate mofetil (MMF) (Roche, Basel, Switzerland) for GVHD prophylaxis. CSA was started [3 mg/(kg.d), i.v.] on day-1 and continued until patients were able to take CSA orally with trough level at 150-250 ng/mL for at least one month. CSA was discontinued on day 180 or before if feasible. MMF was administered orally three times a day from day +1 to +28 to a total dose of 30 mg/kg. Myeloid engraftment was defined as the first day of the three consecutive days during which absolute neutrophil count (ANC) exceeded 0.5×109/L. Platelet engraftment was defined as the first day of the seven consecutive days during which the platelet count exceeded 20×109/L without transfusion support. Pre-engraftment syndrome (PES) was defined as unexplained fever higher than 38.3 °C without confirmed infection and unresponsive to antimicrobial agents and/or unexplained erythematous skin rash occurring prior to neutrophil engraftment.
PCR for CMV
Quantitative real-time PCR (RT-PCR) was performed using a TaqMan (ABI)-based method (17). Briefly, DNA was extracted from 200 µL of whole blood using a Qiagen extraction kit. The primers for CMV DNA amplification included [5'-GAGGACAACGAAATCCTGTTGGGCA-3' (gB1) and 5'-TCGACGGTGGAGATACTGCTGAGG-3' (gB2)] (18). The TaqMan probe [5'-CAATCATGCGTTTGAAGAGGTAGTCCACG-3' (gb-P3)] for the 150 bp product was labeled at the 5' end with 6-FAM and at the 3' end with TAMRA. All samples were analyzed in duplicate, and the average CMV load was used in this manuscript. CMV monitoring was carried out twice weekly for 100 d after the transplantation, and then once weekly for additional 300 d.
Diagnostic criteria of CMV infection and CMV disease
CMV infection was defined as isolation of CMV virus or detection of viral proteins or nucleic acid in any body fluid or tissue specimen (2). CMV disease was defined as clinical and/or laboratory evidence for involvement of the lung, gastrointestinal tract (including the liver), the central nervous system (CNS), or any other organ in subjects with CMV infection (2).
CMV prophylaxis, preemptive therapy, and antiviral therapy
Prophylaxis of CMV infection consisted of acyclovir (5 mg/kg twice daily) beginning on the next day after UCBT and continuing until CMV infection occurred or for 34 d, whichever came first. The preemptive antiviral therapy (ganciclovir 5 mg/kg, q12 h) was initiated in subjects with >1,000 copies of CMV DNA. Patients with agranulocytosis (neutrophils <0.5×109/L) or unable to tolerate the side effects of ganciclovir received foscarnet (60 mg/kg, q12 h). If CMV infection persisted for >2 weeks after the treatment with either ganciclovir or foscarnet, the two drugs were used in combination, but discontinued if the patient could not tolerate the side effects of these two drugs. Intravenous gamma-globulin (IVIG, 0.4 g/kg daily, for 3-5 d) or an investigational drug (cidofovir or maribavir) was used in patients who did not tolerate the ganciclovir/foscarnet combination. Viral load was monitored twice weekly during antiviral therapy until seroconversion. The antiviral treatment was discontinued upon two consecutive negative reports of CMV DNA test. When CMV disease developed during the preemptive therapy, ganciclovir and foscarnet were given in combination with IVIG (0.4 g/kg daily, for 3-5 d).Upon drug resistance to ganciclovir (rising viral load for more than two weeks or development of CMV disease), foscarnet was added. Upon foscarnet resistance, IVIG (0.4 g/kg daily, for 3-5 d) or an investigational drug (cidofovir or maribavir) was used.
Statistical analysis
Continuous variables are expressed as median and range. Differences of baseline characteristics between the groups were analyzed with the Chi-square test or Fisher’s exact test (both 2-sided). Post-transplantation CMV infection was analyzed with a rank order test. Only risk factors associated with the development of CMV infection upon univariate analysis were included in the multivariate Cox regression analysis. The cutoff value for continuous variables was selected based on the observed median (except for age, which was divided into <14 vs. 14 years and older). Overall survival (OS) was defined as the time from UCBT to death or to the last observation. Event-free survival (EFS) was defined as the time from UCBT to relapse, death or the last observation. The OS and EFS were analyzed using the log-rank test. Relapse was defined by morphologic evidence of disease in peripheral blood, bone marrow or extramedullary sites. Treatment-related mortality (TRM) was defined as death from all causes except for relapse. All statistical analyses were carried out using SPSS 16.0 (SPSS Inc., Chicago, IL, USA). A P value <0.05 was considered statistically significant.
Results
Rate of CMV infection and CMV disease
Out of the 176 patients receiving UCBT, 125 (71%) developed CMV infection (Table 2). The median onset was 32 d (range, 18-125 d) from the transplantation. Four patients developed CMV disease (n=3 for CMV pneumonia; n=1 for CMV colitis).
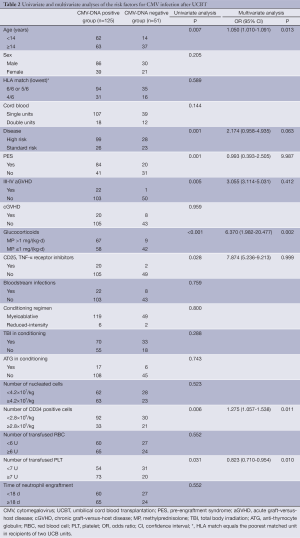
Full table
Risk factors for CMV infection
Demographic data and clinical characteristics of the 125 patients who developed CMV infection were compared to that of the 51 patients who did not develop CMV infection (Table 2). A univariate analysis indicated an association of CMV infection with younger age, high-risk disease, PES, grade III-IV acute GVHD (aGVHD), the use of glucocorticoids [methylprednisolone at >1 mg/(kg.d)], the use of CD25 and tumor necrosis factor-alpha (TNF-α) receptor inhibitor, lower amount of CD34+ cell infusion, and higher number of platelet infusion. A multivariate analysis confirmed the association of CMV infection with younger age, the use of glucocorticoids, lower number of CD34+ cells, and higher number of platelet infusion. CMV infection was not associated with the use of TBI or ATG in the conditioning regimen, the dosage of infused total nucleated cells, gender, weight, UCB units, or HLA match.
Therapeutic outcomes
Out of the 126 patients who developed CMV infection after the transplantation, seroconversion was achieved in 121 cases after treatment, with a median duration of 14 d (range, 7-84 d): 61 cases with ganciclovir alone, 25 cases with foscarnet alone, 18 cases with ganciclovir plus foscarnet, and 17 cases with IVIG or investigational drugs. Four patients developed full-blown CMV disease. The three subjects who developed CMV pneumonia died of respiratory failure. The patient with CMV colitis was cured with ganciclovir and IVIG.
Treatment-related toxicity (TRT)
In the 34 patients receiving preemptive therapy, the rate of total TRT was 67.6% (23/34). Since the preferred drug was ganciclovir, the most common toxicity was neutropenia. The rate of neutropenia (neutrophil count <0.5×109/L) was 35.3% (12/34). Four patients (2.3%) switched to another regimen due to the side effects of ganciclovir and foscarnet. No patient died of toxicity of the antiviral agents.
CMV infection and C-reactive protein (CRP)
Prior to the conditioning therapy, median serum CRP was 4.5 mg/L (range, 2.0-8.9 mg/L). At the onset of CMV infection, the median CRP was 15.8 mg/L (range, 9.7-37.2 mg/L). Peak CRP during CMV infection was 35.6 mg/L (range, 21.2-65.8 mg/L). After the antiviral therapy that resulted in seroconversion, serum CRP reduced to 4.9 mg/L (range, 3.0-11.5 mg/L). Patients who developed CMV disease had much higher peak serum CRP (median, 145 mg/L; range, 113-190 mg/L).
CMV infection and survival
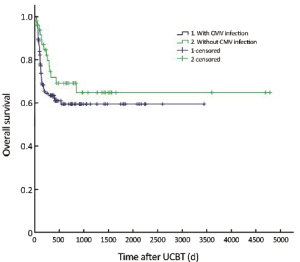
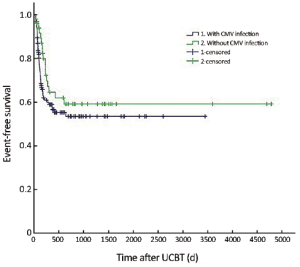
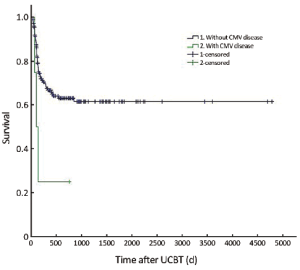
The median follow-up was 756 d (31-4,786 d) after the transplantation. For the subjects who were still alive at the time of preparation of this manuscript, the median follow-up was 858 d (range, 210-4,786 d). The cumulative mortality rate (CMR) was 40.8% (51/125) in the patients with CMV infection and 27.5% (14/51) in those without CMV infection (P=0.096). The 5-year OS was 59.4% and 64.8% in the patients with and without CMV infection, respectively (P=0.194) (Figure 1). The 5-year EFS was 53.4% and 59.1% in the patients with and without CMV infection, respectively (P=0.226) (Figure 2). CMV disease was associated with high rate (3/4) of death (Figure 3).
CMV infection and relapse
Relapse was evident in 13 of the 125 patients (10.4%) with CMV infection and 7 of the 51 CMV-negative patients (13.7%; P=0.528).
Discussion
CMV infection occurs at a high rate (typically at 70%) after UCBT, but varies considerably across transplantation centers (14,19). With prudent use of antiviral prophylaxis and preemptive antiviral therapy, the incidence of CMV disease could be decreased by 15-25% (20). The incidence of CMV infection in the study was comparable to that reported in the literature. The incidence of CMV disease, however, was much lower than previously reported. This finding likely reflects the preemptive therapy in the current study. When the viral DNA copies were below 1,000, the dosage of immunosuppressants should be reduced while not increasing the risk of GVHD. When the viral load exceeds 1,000/mL, antiviral treatment is warranted (21). At present, the main antiviral agents are ganciclovir and foscarnet. Newer antiviral drugs (e.g., maribavir and cidofovir) have not been widely used, and their effects on CMV infection and CMV disease after UCBT are unclear (22). Another possible contributor to the low incidence of CMV disease in the current study was the conservative use of ATG conditioning regimen. The three most commonly used conditioning regimens in the current study were: TBI + cytarabine (Ara-c) + cyclophosphamide (CY), fludarabine (Flu) + busulfan (BU) + CY, and Ara-c + BU + CY. CSA in combination with MMF was used to prevent GVHD in the current study due to low immunogenicity of the UCB.
Immunosuppression results in higher incidence of CMV infection (23). Consistently, the use of high-dose glucocorticoids was a risk factor for CMV infection in the current study. A novel finding of the current study is the association of PES with CMV infection in UCBT patients, but such an association may be secondary to higher dose of methylprednisolone needed for PES (24). A previous study from our group indicated that the risk factors of PES were myeloablative conditioning regimen and younger age (25). As a result, the association of PES with CMV infection could also be due to the younger age.
Similar to a previous study by Matsumura et al. (11), CMV infection was associated with the number of infused CD34+ cells but not the total number of nucleated cells or CD3+ cells in the current study.
Transplant-related complications and transplant-related mortality were much higher in high-risk patients compared with standard-risk patients. The increasing need of platelet transfusions in these patients increases the risk of CMV infection. Because CMV invades the recipients through infused donor granulocytes, even CMV-negative blood products carry a risk of transmitting CMV (26). Filtering out leukocytes for platelet infusion could reduce the risk of CMV infection (27).
The acute-phase protein CRP is produced mainly by hepatocytes, and correlates with inflammation. Not surprisingly, we identified a close association between CRP and CMV infection. However, elevated CRP is a non-specific indicator of inflammation and infection, and by no means a specific indication of CMV infection. Also, patients with CMV disease had much higher CRP levels in comparison to those with only CMV infection. Thus, patients with grossly elevated serum CRP after UCBT should be suspected of having CMV disease.
The results from the current study seem to suggest that CMV infection alone does not affect the OS and EFS in UCBT patients. CMV infection seems to offer certain advantages based on literature. For example, a previous study indicated that a diverse polyclonal CMV-specific T cells derived from the UCB graft could be primed by the viral antigens as early as day 42 after UCBT, but fail to achieve sufficient numbers in vivo to control CMV reactivation (28). CMV infection could drive NK cell development after UCBT, and enhance immunity (29). Another study even suggested that early human CMV replication after transplantation is associated with decreased risk for relapse and enhanced virus-versus-leukemia effect in acute myeloid leukemia patients (30). Consistent with this notion, we found a trend for lower (but not statistically significant) relapse rate in CMV-infection patients (10.4%) than CMV-negative patients (13.7%).
In summary, CMV infection itself does not affect the survival of UCBT patients, but progression into CMV disease is highly fatal. We suspect the relatively low incidence of CMV disease is at least partly due to preemptive antiviral therapy.
Acknowledgements
This research was supported by the National Natural Science Fund of China (81250001), the Twelfth Five-year Science and Technology Project in Anhui Province (11010402164), Anhui Province Science and Technology Leader of Scientific Research Fund and Anhui Provincial ‘115’ Industrial Innovation Program [2009].
Disclosure: The authors declare no conflict of interest.
References
- Gratama JW, Boeckh M, Nakamura R, et al. Immune monitoring with iTAg MHC Tetramers for prediction of recurrent or persistent cytomegalovirus infection or disease in allogeneic hematopoietic stem cell transplant recipients: a prospective multicenter study. Blood 2010;116:1655-62. [PubMed]
- Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis 2002;34:1094-7. [PubMed]
- Benito AI, Diaz MA, González-Vicent M, et al. Hematopoietic stem cell transplantation using umbilical cord blood progenitors: review of current clinical results. Bone Marrow Transplant 2004;33:675-90. [PubMed]
- Cohen Y, Nagler A. Umbilical cord blood transplantation–how, when, and for whom? Blood Rev 2004;18:167-79. [PubMed]
- Brown JA, Boussiotis VA. Umbilical cord blood transplantation: basic biology and clinical challenges to immune reconstitution. Clin Immunol 2008;127:286-97. [PubMed]
- Gutman JA, Leisenring W, Appelbaum FR, et al. Low relapse without excessive transplant-related mortality following myeloablative cord blood transplantation for acute leukemia in complete remission: a matched cohort analysis. Biol Blood Marrow Transplant 2009;15:1122-9. [PubMed]
- Baek HJ, Kook H, Han DK, et al. Hematopoietic stem cell transplantation in children with leukemia: a single institution experience with respect to donors. J Korean Med Sci 2011;26:1548-55. [PubMed]
- Takahashi S, Iseki T, Ooi J, et al. Single-institute comparative analysis of unrelated bone marrow transplantation and cord blood transplantation for adult patients with hematologic malignancies. Blood 2004;104:3813-20. [PubMed]
- Takahashi S, Ooi J, Tomonari A, et al. Comparative single-institute analysis of cord blood transplantation from unrelated donors with bone marrow or peripheral blood stem-cell transplants from related donors in adult patients with hematologic malignancies after myeloablative conditioning regimen. Blood 2007;109:1322-30. [PubMed]
- Smith AR, Baker KS, Defor TE, et al. Hematopoietic cell transplantation for children with acute lymphoblastic leukemia in second complete remission: similar outcomes in recipients of unrelated marrow and umbilical cord blood versus marrow from HLA matched sibling donors. Biol Blood Marrow Transplant 2009;15:1086-93. [PubMed]
- Matsumura T, Narimatsu H, Kami M, et al. Cytomegalovirus infections following umbilical cord blood transplantation using reduced intensity conditioning regimens for adult patients. Biol Blood Marrow Transplant 2007;13:577-83. [PubMed]
- Walker CM, van Burik JA, De For TE, et al. Cytomegalovirus infection after allogeneic transplantation: comparison of cord blood with peripheral blood and marrow graft sources. Biol Blood Marrow Transplant 2007;13:1106-15. [PubMed]
- Tomonari A, Takahashi S, Ooi J, et al. Impact of cytomegalovirus serostatus on outcome of unrelated cord blood transplantation for adults: a single-institute experience in Japan. Eur J Haematol 2008;80:251-7. [PubMed]
- Tomonari A, Takahashi S, Ooi J, et al. Preemptive therapy with ganciclovir 5 mg/kg once daily for cytomegalovirus infection after unrelated cord blood transplantation. Bone Marrow Transplant 2008;41:371-6. [PubMed]
- Beck JC, Wagner JE, DeFor TE, et al. Impact of cytomegalovirus (CMV) reactivation after umbilical cord blood transplantation. Biol Blood Marrow Transplant 2010;16:215-22. [PubMed]
- Huang XJ, Wang Y, Liu DH, et al. Modified donor lymphocyte infusion (DLI) for the prophylaxis of leukemia relapse after hematopoietic stem cell transplantation in patients with advanced leukemia--feasibility and safety study. J Clin Immunol 2008;28:390-7. [PubMed]
- Fox JC, Kidd IM, Griffiths PD, et al. Longitudinal analysis of cytomegalovirus load in renal transplant recipients using a quantitative polymerase chain reaction: correlation with disease. J Gen Virol 1995;76:309-19. [PubMed]
- Fox JC, Griffiths PD, Emery VC. Quantification of human cytomegalovirus DNA using the polymerase chain reaction. J Gen Virol 1992;73:2405-8. [PubMed]
- Milano F, Pergam SA, Xie H, et al. Intensive strategy to prevent CMV disease in seropositive umbilical cord blood transplant recipients. Blood 2011;118:5689-96. [PubMed]
- Mori T, Okamoto S, Watanabe R, et al. Dose-adjusted preemptive therapy for cytomegalovirus isease based on real-time polymerase chain reaction after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 2002;29:777-82. [PubMed]
- Mikulska M, Raiola AM, Bruzzi P, et al. CMV infection after transplant from cord blood compared to other alternative donors: the importance of donor-negative CMV serostatus. Biol Blood Marrow Transplant 2012;18:92-9. [PubMed]
- Drew WL, Miner RC, Marousek GI, et al. Maribavir sensitivity of cytomegalovirus isolates resistant to ganciclovir, cidofovir or foscarnet. J Clin Virol 2006;37:124-7. [PubMed]
- Boeckh M, Ljungman P. How we treat cytomegalovirus in hematopoietic cell transplant recipients. Blood 2009;113:5711-9. [PubMed]
- Kanda J, Kaynar L, Kanda Y, et al. Pre-engraftment syndrome after myeloablative dual umbilical cord blood transplantation: risk factors and response to treatment. Bone Marrow Transplant 2013;48:926-31. [PubMed]
- Wang X, Liu H, Li L, et al. Pre-engraftment syndrome after unrelated donor umbilical cord blood transplantation in patients with hematologic malignancies. Eur J Haematol 2012;88:39-45. [PubMed]
- Hersman J, Meyers JD, Thomas ED, et al. The effect of granulocyte transfusions on the incidence of cytomegalovirus infection after allogeneic marrow transplantation. Ann Intern Med 1982;96:149-52. [PubMed]
- Nichols WG, Price TH, Gooley T, et al. Transfusion-transmitted cytomegalovirus infection after receipt of leukoreduced blood products. Blood 2003;101:4195-200. [PubMed]
- McGoldrick SM, Bleakley ME, Guerrero A, et al. Cytomegalovirus-specific T cells are primed early after cord blood transplant but fail to control virus in vivo. Blood 2013;121:2796-803. [PubMed]
- Francois S, Peng J, Schwarz T, et al. NK cells improve control of friend virus infection in mice persistently infected with murine cytomegalovirus. Retrovirology 2013;10:58. [PubMed]
- Elmaagacli AH, Steckel NK, Koldehoff M, et al. Early human cytomegalovirus replication after transplantation is associated with a decreased relapse risk: evidence for a putative virus-versus-leukemia effect in acute myeloid leukemia patients. Blood 2011;118:1402-12. [PubMed]


