Does an extended mediastinal lymphadenectomy improve outcome after R0 resection in lung cancer?
Introduction
The 5-year survival rate for lung cancer has improved significantly from 12.3% in 1975-1977 to 16.9% in 2002-2008 (1). This progress may be attributed to the widespread application of multidisciplinary treatments for lung cancer. Meantime, surgery remains a key treatment in the medical interventions for lung cancer, especially for those with early stage disease. Surgical techniques have evolved rapidly in the past several decades. However, substantial differences are still observed in the quality evaluation standards for lymphadenectomy defined by individual academic societies and research groups.
Successful lung cancer surgery requires complete resection, removal of an adequate anatomic volume of lung tissue and removal of the draining lymph nodes in the lung and mediastinum (2). Lymph node examination is directly linked with the quality of pathological staging. Sufficient tissue supplies will secure the accurate staging based on acceptable lymph node clearance procedures. But it still needs to investigate whether a more radical extent of lymph node clearance could yield a better survival and meanwhile recovery process remained similar. Thus we designed this retrospective study to investigate the impact of quality improvement of lymphadenectomy on the outcome of lung cancer patients treated with R0 resection.
Patients and methods
Subjects
From September, 2003 to December, 2007, consecutive patients of stage I, stage II and resectable stage IIIa non-small cell lung cancer (NSCLC) were eligible in the study protocol. Patients diagnosed with pulmonary malignancies other than primary lung cancer were excluded from the study. Cases with N3 or bulky N2 disease (3) after induction chemotherapy were excluded.
All operations were performed by a single surgical group. Pulmonary function testing and cardiac evaluation were required as preoperative assessments. Brain magnetic resonance imaging (MRI) or computed tomography (CT), positron emission tomography/CT (PET/CT) scans, bone scintigraphy, and abdominal and supraclavicular ultrasound scanning were used to rule out potential metastatic lesions or N3 disease. Pulmonary resections, including wedge resection, lobectomy, bilobectomy, sleeve-lobectomy and pneumonectomy were selected on the basis of tumor location. Lymph-node stations were defined according to the International Association for the Study of Lung Cancer (IASLC) manual (4). The status of the residual tumor after surgical treatment was defined as three categories: R0 resection (no residual tumor present), R1 resection (microscopically residual tumor) and R2 resection (macroscopically residual tumor) (5).
With the gradual acceptance of systematic mediastinal lymph node dissection (SMLD) in our clinical practice, a transition of surgical techniques on mediastinal lymphadenectomy occurred during the period from December, 2005 to January, 2006. Therefore, the entire patient cohort was artificially divided into two subgroups, according to the extent of mediastinal lymphadenectomy. From September, 2003 to December, 2005, the surgeons resected the mediastinal lymph nodes based on palpation and inspection, which was defined as Control group. From January, 2006 to December, 2007, SMLD was performed using a standard procedure according to IASLC (4) and this group was defined as Research group.
Methods
Briefly for SMLD, all fatty tissue and lymphatic tissue in the right upper mediastinum (No. 2 and No. 4 on the right) were removed en bloc from the superior vena cava anteriorly, to the trachea posteriorly, and from the takeoff of the right upper lobe inferiorly, to the caudal border of the innominate artery superiorly. The lymph nodes located between the trachea and the esophagus (No. 3P), and the lymph nodes anterior and medial to the superior vena cava at the insertion of the azygous vein (No. 3A) were also dissected. On the left side, dissection of No. 6 was carried out by the removal of all fat pads and lymph nodes anterior and lateral to the ascending aorta and the aortic arch. No. 5 was dissected by removal of the fatty-lymphatic tissues lateral to the ligamentum arteriosum and proximal to the first branch of the left pulmonary artery. No. 4 was dissected from the left side of the trachea medially to the ligamentum arteriosum laterally, and from the upper margin of the aortic arch to the takeoff of the left main bronchus inferiorly. For No. 7, surgeons ensured that the carina, and the left and the right main bronchi were exposed and free of fatty tissue after dissection. Lymph nodes adjacent to the inferior pulmonary ligament were also removed.
The tissues and lymph nodes were sent for paraffin embedding and routine pathologic analysis. The lymph nodes were bisected along their longitudinal axis and submitted for total microscopic evaluation. Small nodes (0.4 cm or less) were submitted without bisection. A single hematoxylin-eosin (H&E)-stained slide was produced from each block.
Statistical analysis
The Institutional Review Board of the Peking University Cancer Hospital approved this retrospective study. The requirement of patient consent was waived. Comparison of the outcome between Control group and Research group was analyzed using the log-rank test and Kaplan-Meier curves were generated for overall survival (OS) and disease-free survival (DFS). Multivariate Cox regression analyses were used to determine the factors significantly associated with survival. Values were expressed as the
Results
Three hundred and twenty-five cases of primary lung cancer were treated during the period of investigation, comprising 290 cases of R0 resection, 10 cases of R1 resection and 25 cases of R2 resection. R1 or R2 resection was excluded from further analysis. In addition, five R0 cases were also excluded from the analysis, including three patients who died within 30 d of surgery, and two patients who died due to non-cancerous causes within the postoperative multi-modality treatment period (one sudden death due to bronchopleural fistula; one severe infection due to grade 4 leukocytopenia during adjuvant chemotherapy). Follow-up ended on June 30, 2011; seven cases were lost during the investigation period and thus excluded from the study (two in Control group and five in Research group). Ultimately, a total of 278 patients were analyzed for OS in this study and 242 cases were analyzed for DFS (36 cases were discarded from DFS analysis because their recurrence information was lost). After a median follow-up of 48.95 months (range, 3.7-92.3 months), 113 patients (40.65%) had died. The 5-year OS rates for the whole group, pathological stage I (n=112), stage II (n=65) and stage IIIa (n=97) were 58.9±3.2%, 74.2±4.7%, 64.8±6.1% and 38.8±5.3%, respectively.
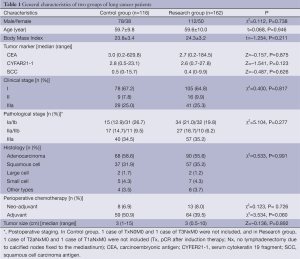
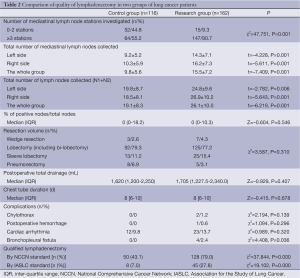
The general characteristics of the patients in Control group and Research group are summarized in Table 1. One hundred and sixteen cases were enlisted in Control group and 162 cases in Research group. The two groups were equally matched in terms of gender, age, Body Mass Index (BMI), pathological staging, histology, and the ratio of perioperative chemotherapy. To compare the quality of lymphadenectomy and surgical care between the two groups, the following parameters were analyzed: the extent of lymph node clearance (number of mediastinal node stations and number of lymph nodes), the resection volume, and the postoperative recovery process and common complications (Table 2).
Full table
Full table
Significant differences were observed in the number of mediastinal lymph node stations investigated (more than 3 N2 stations investigated, 55.2% in Control group vs. 90.7% in Research group, P=0.001), the total number of lymph nodes harvested (19.1±8.3 in Control group vs. 26.1±10.0 in Research group, P=0.000), and the total number of N2 nodes collected (9.8±5.6 in Control group vs. 15.5±7.2 in Research group, P=0.000). There was no significant difference in other quality-related items, such as the proportion of metastatic nodes, resection volume, or recovery and common complications (chylothorax, postoperative hemorrhage, and cardiac arrhythmia).
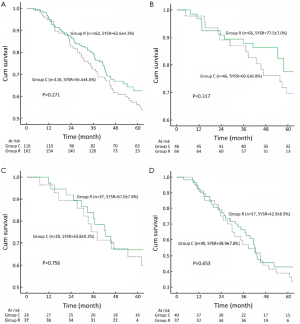
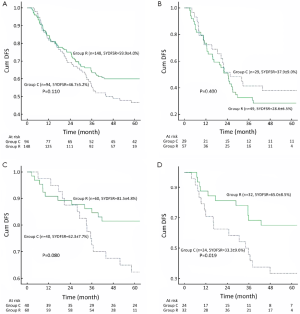
The Kaplan-Meier OS curves are depicted in Figure 1. Control group had a similar outcome compared with Research group. The 5-year OS rates were 56.4±4.6% in Control group and 62.6±4.3% in Research group (P=0.271, Figure 1A). Significant differences in OS were not observed between Control group and Research group when the patients were stratified into stage I (Figure 1B), stage II (Figure 1C) and stage IIIa (Figure 1D). The difference in DFS between Control group and Research group was not significant in the whole group (Figure 2A) and in stage IIIa patients (Figure 2B). However, DFS in stage I (Figure 2C) and stage II (Figure 2D) patients tended to be longer in Research group.
In multivariate analysis, the significant factors associated with OS were TNM classification, gender, and histology, as shown in Table 3. Meanwhile, TNM classification and histology were significant factors associated with DFS. Quality-related factors, such as the extent of mediastinal lymph node clearance, the number of mediastinal node stations investigated and the resection volume were not significantly correlated with OS or DFS.
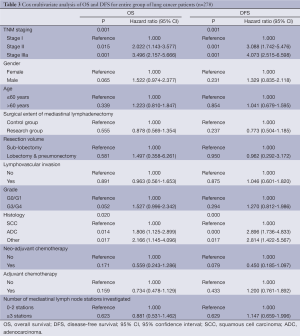
Full table
Discussion
The quality of lymphadenectomy is a comprehensive concept, in which the surgeons’ understanding and practice form a solid foundation. High quality of lymph node investigation is supposed to lead to more accurate staging. In this retrospective study, 75.9% of the whole group received lymph node resection at more than three N2 stations; the median number of mediastinal lymph nodes and total lymph nodes collected was 13 and 22, respectively. Research group received a more radical extent of mediastinal lymphadenectomy compared with Control group, as indicated by investigation of a larger number of N2 stations and harvesting of more lymph nodes (N2 or total). More cases attaining National Comprehensive Cancer Network (NCCN) (6) or IASLC criteria for qualified lymphadenectomy occurred in the Research group. Although the Kaplan-Meier curves on OS and DFS both showed a tendency toward slightly improved outcome, they were not statistically different. These results suggest that extended mediastinal lymphadenectomy may not yield a potential therapeutic benefit.
Lymphadenectomy is considered to be a key component of surgical quality; however, several procedures are available, such as SMLD, systematic sampling (SS) and random sampling (7). In stage I NSCLC, lymphadenectomy was associated with increased OS and DFS, compared with that of patients who did not receive lymphadenectomy (8). Gajra et al. indicated that SMLD and SS could lead to the collection of a larger number of lymph nodes in stage I NSCLC, and these techniques were associated with improved survival (9). Thus, a certain extent of lymphadenectomy, such as SMLD or SS, seems to secure a survival benefit in early stage lung cancer. However, debate still exists regarding the optimal method for qualified mediastinal lymphadenectomy in lung cancer. Doddoli et al. suggested that, compared with sampling, SMLD improved survival in a retrospective study of stage I NSCLC (10). The largest randomized control trial (RCT) in this field, the Z0030 trial, demonstrated that SMLD did not improve survival over SS in patients with no mediastinal and hilar lymph node metastasis (11). Two other smaller trials also suggested that SMLD might not affect OS in lung cancer patients (12,13). Only one RCT trial has demonstrated a survival benefit for SMLD (over sampling) in a cohort of 532 cases; however, the sampling procedure, defined as the removal of suspected nodes larger than 1 cm or hard nodes, was more likely to be random sampling (14).
So far there are no prospective studies on how to perform qualified mediastinal lymphadenectomy for more advanced disease, especially at stage II or IIIa. In this study, more extended lymphadenectomy may not achieve an OS benefit in stage II, even though the PFS in Research group was better than that of Control group, which implies the role of multidisciplinary treatment in this group. The latest American College of Chest Physicians (ACCP) guideline also suggests every patient should have systematic mediastinal lymph node sampling and SMLD may be performed without increased morbidity in early stage lung cancer (15). Thus, both methods may be feasible in dealing with mediastinal lymphadenectomy for clinical early stages. However, for stage IIIa, it is still unknown that whether SMLD could be superior to SS. But at least, complete resection should be advised in single zone N2 node involvement or mediastinal downstage after induction therapy (16).
As SS yielded similar rates of survival and comorbidity compared with SMLD, this raises the question of whether SS represents the lowest, acceptable standard for curative resection in lung cancer. Unfortunately, the evidence is not yet strong enough to reach a conclusion, especially for stage II and stage IIIa patients. In a previous study, we reported that the negative predictive value of SS, in terms of mediastinal lymph node metastasis, was 86.8% for the right side and 95% for the left side. Additionally, the diagnosis after SS was pathologically understaged in 8.2% of cases, compared with staging after SMLD (17). Therefore, further investigation is needed to clarify the indication of SS, which may yield a similar outcome at certain stages for operable lung cancer.
Retrospective analysis and non-randomization are the major limitations of this study. However, to explore the quality improvement of lymphadenectomy, randomization design might put the Control group at risk of insufficient lymph node clearance. It is reasonable to perform the clinical trial in early stage disease. But retrospective analysis is suitable for more advanced disease. The Z0030 trial (14) has provided the strongest evidence to date to suggest that SMLD does not improve survival in patients with stage I lung cancer. However, more studies are needed to explore the effect of lymphadenectomy on the outcome for the advanced lung cancer patients. Sample size was another limitation of the study. A larger sample size study and multicenter participation might reduce the bias for this purpose.
In summary, more radical mediastinal lymphadenectomy may not lead to an improved oncological outcome for lung cancer treated with R0 resection.
Acknowledgements
This study was supported partially by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA06020101), the National Natural Science Foundation (No. 81350028), the National High Technology Research and Development Program of China (863 Program, No. 2012AA02A502), and the Beijing Municipal Science & Technology Commission (No. Z111107067311018).
Disclosure: The authors declare no conflict of interest.
References
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2009 (Vintage 2009 Populations), National Cancer Institute. Bethesda, MD. Available online: http://seer.cancer.gov/csr/1975_2009_pops09/. Accessed April 2012. Non-small cell lung and bronchus cancer (invasive) survival rates, by race, sex, diagnosis year, stage and age.
- Scott WJ, Howington J, Feigenberg S, et al. Treatment of non-small cell lung cancer stage I and stage II: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:234S-42S.
- Robinson LA, Ruckdeschel JC, Wagner H Jr, et al. Treatment of non-small cell lung cancer-stage IIIA: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:243S-65S.
- Peter Goldstraw. IASLC Staging Manual in Thoracic Oncology. 1st ed. Orange Park: Editorial Rx Press, 2009:66-80.
- Rami-Porta R, Wittekind C, Goldstraw P, et al. Complete resection in lung cancer surgery: proposed definition. Lung Cancer 2005;49:25-33. [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Non-Small Cell Lung Cancer (v.3.2012). Available online: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#nscl, accessed April 11.
- Osarogiagbon RU, Allen JW, Farooq A, et al. Objective review of mediastinal lymph node examination in a lung cancer resection cohort. J Thorac Oncol 2012;7:390-6. [PubMed]
- Varlotto JM, Recht A, Nikolov M, et al. Extent of lymphadenectomy and outcome for patients with stage I nonsmall cell lung cancer. Cancer 2009;115:851-8. [PubMed]
- Gajra A, Newman N, Gamble GP, et al. Effect of number of lymph nodes sampled on outcome in patients with stage I non-small cell lung cancer. J Clin Oncol 2003;21:1029-34. [PubMed]
- Doddoli C, Aragon A, Barlesi F, et al. Does the extent of lymph node dissection influence outcome in patients with stage I non-small-cell lung cancer? Eur J Cardiothorac Surg 2005;27:680-5. [PubMed]
- Darling GE, Allen MS, Decker PA, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg 2011;141:662-70. [PubMed]
- Izbicki JR, Passlick B, Pantel K, et al. Effectiveness of radical systematic mediastinal lymphadenectomy in patients with resectable non-small cell lung cancer: results of a prospective randomized trial. Ann Surg 1998;227:138-44. [PubMed]
- Passlick B, Kubuschock B, Sienel W, et al. Mediastinal lymphadenectomy in non-small cell lung cancer: effective¬ness in patients with or without nodal micrometastases - results of a preliminary study. Eur J Cardiothorac Surg 2002;21:520-6. [PubMed]
- Wu YL, Huang ZF, Wang SY, et al. A randomized trial of systematic nodal dissection in resectable non-small cell lung cancer. Lung Cancer 2002;36:1-6. [PubMed]
- Howington JA, Blum MG, Chang AC, et al. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e278S-313S.
- Bakir M, Fraser S, Routledge T, et al. Is surgery indicated in patients with stage IIIa lung cancer and mediastinal nodal involvement? Interact Cardiovasc Thorac Surg 2011;13:303-10. [PubMed]
- Wu N, Yan S, Lv C, et al. Comparison of systematic mediastinal lymph node dissection versus systematic sampling for lung cancer staging and completeness of surgery. J Surg Res 2011;171:e169-73. [PubMed]


