Prognostic role of lymph node metastasis in early gastric cancer
Introduction
Early gastric cancer (EGC) has been defined as a gastric carcinoma confined to the mucosa and/or submucosa, regardless of the size or presence of lymph node metastasis. EGCs have a low incidence of lymph node metastasis and a favorable outcome after surgery (1,2). Treatment options for EGC include endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD), wedge resection, laparoscopically assisted gastrectomy and open gastrectomy (3,4). Endoscopic dissection is a widely accepted treatment for EGC in patients with appropriate criteria, of which the absence of lymph node metastasis is a necessary prerequisite (5,6). Patients with EGC generally have an excellent prognosis after curative resection (R0), with 5- and 10-year survival rates of more than 90% and 85-90%, respectively (7-9). However, recurrence can still occur after curative resection of EGC, with a rate of 1.3-13.8% reported in previous studies (10,11). Lymph node metastasis is one of the most important factors in determining the prognosis of patients with EGC (12,13). Thus, identifying those patients with lymph node metastasis is crucial for the selection of appropriate treatments and the implications for prognosis.
In the light of these considerations, we carried out this study to clarify the relationship between clinicopathological features and the incidence of lymph node metastasis and to propose the potential indications of lymph node metastasis for prognosis purposes and for the likelihood of tumor recurrence/metastasis in EGC patients.
Materials and methods
Study population
We enrolled 226 patients with EGC pathologically proven after gastrectomy with lymph node dissection in Beijing Cancer Hospital between December 1996 and December 2011. The standard operative procedures were performed for EGC: distal subtotal or total gastrectomy, depending on the location of gastric cancer, with D2 or more extended lymphadenectomy. The inclusion criteria for such procedures were as follows: the gastric tumor invasion was limited to the mucosa or submucosa; no less than D1 lymph node dissection was performed; and no patient had received neoadjuvant therapy before surgery. We gathered patient data relating to: age and sex, tumor location, macroscopic type, size, histological type and depth of invasionand extent of lymphovascular invasion. The classification of the dissected lymph nodes was verified by surgeons reviewing the excised specimens after surgery based on the TNM Classification of Malignant Tumors (7th Edition) of the Union for International Cancer Control (UICC)-pN category (pN0, no metastasis; pN1, 1-2 metastatic lymph nodes; pN2, 3-6 metastatic lymph nodes; pN3, 37 metastatic lymph nodes).The depth of tumor invasion was classified as mucosal or submucosal invasion, and the maximum diameter of the tumor was recorded as the tumor size. We classified the carcinomas into three macroscopic types: protruded type [(I), superficial type (II, including elevated (IIa), flat type (IIb) and depressed type (IIc)], and excavated type (III). Follow up of the entire study population was conducted until death or the follow-up date, by means of outpatient clinic consultation and/or communication with patients and their relatives via telephone or letter. We recorded data concerning clinical and pathological features and all follow-up information, and then calculated 5- and 10-year survival rates. This study was approved by the Ethics Committee of the Beijing Cancer Hospital, and informed consent was obtained from all individuals.
Statistical analysis
We performed all the statistical analyses using the SPSS 20.0 statistical package (SPSS Inc., Chicago, IL, USA). We analyzed associations between lymph node metastasis and clinicopathological parameters using the chi-square test (or Fisher’s exact test when appropriate). In multivariate analysis, logistic regression analysis was applied to identify the independent clinicopathological factors correlating to lymph node metastasis. We estimated the probability of lymph node metastasis with 95% confidence interval (95% CI) based on binominal distribution. Overall survival rates were determined using the Kaplan-Meier estimator, an event being defined as death from cancer-related cause. We used the log-rank test to identify differences between the survival curves, and we performed multivariate prognosis analysis using the Cox proportional hazards model, selecting a forward stepwise procedure. Hazard ratio and 95% CI were calculated. We statistically analyzed the relationship between lymph node metastasis and tumor recurrence/metastasis using the chi-square test (or Fisher’s exact test when appropriate). For all analyses, P<0.05 was considered statistically significant.
Results
Clinicopathological characteristics of EGC
We analyzed the overall data from the 226 patients, comprising 156 men and 70 women. The mean age was 59 years old (range, 24-82 years). The mean number of metastatic lymph nodes was 1 (range, 1-25). Among the EGC patients, 17 (7.5%) were histologically shown to have lymph node metastasis, including 11 (4.8%) categorized as N1, 2 (0.9%) as N2, and 4 (1.8%) as N3, and 209 (92.5%) had no lymph node metastasis (N0).
Lymph node metastasis was significantly associated with tumor differentiation, lymphovascular invasion and depth of invasion (P=0.022, 0.000, and 0.001, respectively). Cases of undifferentiated carcinoma, lymphovascular and/or submucosal invasion were associated with higher lymph node metastasis. Tumors larger than 2 cm and excavated type (III) tumors had a higher probability of lymph node metastasis, but they did not reach statistical significance (P>0.05). There were no significant differences between gender, age, location and histology characteristics (Table 1).
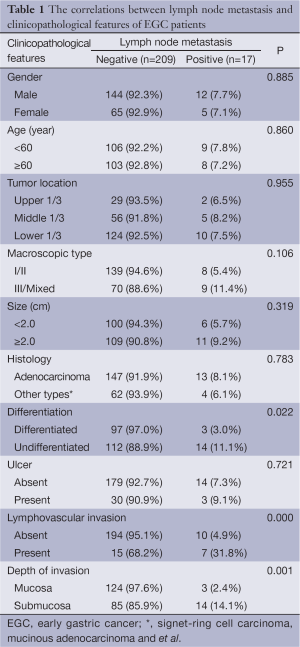
Full table
Further multivariate logistic regression analysis showed that differentiation [P=0.039; relative risk (RR) 2.031; 95% CI, 1.035-3.986], lymphovascular invasion (P=0.003; RR 6.102; 95% CI, 1.846-20.170) and depth of invasion (P=0.035; RR 4.237; 95% CI, 1.107-16.222) were independent risk factors for lymph node metastasis in EGC (Table 2).
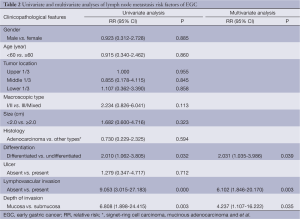
Full table
Survival analyses of EGC patients after surgery
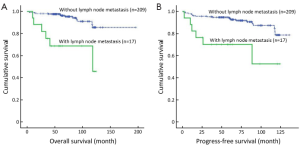
The median survival time was 69.55 months (range, 3.45-195.85 months) in the present study. Survival analysis is shown in Table 3. Of all 226 EGC patients, 207 (91.6%) were still alive in our study. Overall, the 5- and 10-year survival rates were significantly lower in the cases with lymph node metastasis than in those without lymph node metastasis (P=0.000, 68.8% vs. 96.9%, 45.8% vs. 85.6%, respectively, Figure 1A), while the patients with more than 2 lymph node metastases had even lower survival rates (16.7%). In addition, the survival status was also significantly correlated with tumor size (P=0.023; 32.0 cm vs. <2.0 cm; 5-year survival rate, 94.1% vs. 95.5%; 10-year survival rate, 68.7% vs. 91.8%, respectively). However, there were no significant differences in survival rates between different genders, ages, macroscopic types, differentiation, histology, lymphovascular invasion and depth of invasion (all P>0.05). Furthermore, the patients with lymph node metastases had shorter progression-free survival times after surgery than those without lymph node metastases (P=0.000, Figure 1B). Multivariate analysis identified lymph node metastasis (P=0.000; RR 6.451; 95% CI, 2.432-17.111) and tumor size (P=0.041; RR 2.934; 95% CI, 1.044-8.247) as independent prognostic factors for EGC (Table 4).
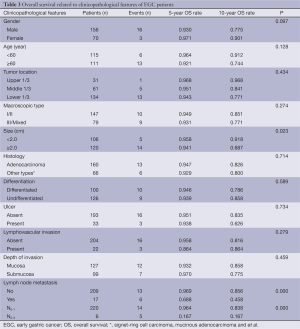
Full table
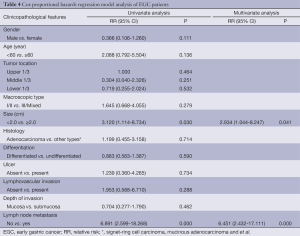
Full table
Clinicopathological features according to recurrence or metastasis after curative surgery
The recurrence or metastasis rate after curative surgery was 6.6% (15/226) in the present study. There were no significant differences in gender, tumor location, tumor size, macroscopic type, differentiation, histology, lymphovascular invasion, and depth of invasion between the recurrence/metastasis group and the non-recurrence/ non-metastasis group. Lymph node metastasis was considered to be a significant factor for cancer metastasis/recurrence (P=0.018, Table 5). Half of the patients with more than 2 lymph node metastases had metastasis/recurrence, as opposed to only 5.3% of those with less than 2 lymph node metastases. Multivariate analysis showed that the status of lymph node metastasis was a significant factor for predicting cancer metastasis or recurrence (P=0.001; RR 18.006; 95% CI, 3.085-105.104).
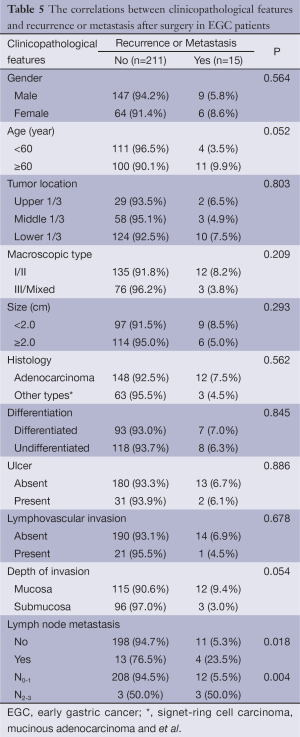
Full table
Discussion
The survival of patients with gastric cancer is still poor although the 5-year survival rate has improved owing to early detection, standardized radical operations and several modified therapeutic modalities (5). Although EGC has an excellent prognosis, previous reports reveal that the prognosis of these patients was mostly affected by lymph node metastasis (13-15). The most important factor to consider when selecting correct treatment modalities for EGC is the presence of lymph node metastasis. According to the increasing number of EGC diagnoses and endoscopic resections, accurate evaluation of the associated risk factors of lymph node metastasis and the status of the lymph nodes in prognosis is critical for subsequent decisions regarding EGC treatment after the initial curative treatment.
In the present study, the incidence of lymph node metastasis was 7.5% among the EGC patients, which is similar to that of an earlier study. Differentiated carcinoma, without lymphovascular or submucosal invasion, had less probability of lymph node metastasis, which was comparable with the findings of previous studies (16,17). Other clinical and pathological factors, such as ulcerated lesions, histological type and tumor size, have been shown to aid in the assessment of risk of nodal metastasis, as in our study (6,18,19). Moreover, the 5- and 10-year overall survival rate or progression-free survival rate and metastasis/recurrence rate were associated with the status of lymph node metastasis, which were consistent with that of earlier studies (10,11). Tumor size is a controversial factor in the prognosis of EGC. Kunisaki et al. noted that tumor size in gastric cancer is a reliable prognostic factor, as we found in the present study (20), so it could be a suitable candidate for use in the staging system. However, other authors suggested that tumor size was not an independent prognostic factor (21). Although previous studies have noted that the recurrence of EGC was significantly higher in patients with submucosal, node-positive and undifferentiated tumors (22), we observed that only lymph node metastasis was the most significant risk factor for tumor recurrence or metastasis after curative operation. However, as Kikuchi et al. (23) reported, in our study submucosal cancers do not recur more frequently than mucosal cancers. Tumor angiogenesis plays a critical role in tumor progression of a variety of malignancies and is crucial in the choice of therapeutic strategy (24). In contrast to angiogenesis in other tumors, previous studies have found that peritumoral lymphatic vessel density was correlated with lymph node metastasis and survival in gastrointestinal carcinoma (25,26), which supports our viewpoint in molecular biology.
Based on our study, EGC patients with undifferentiated carcinoma, lymphovascular and/or submucosal invasion should be carefully assessed for appropriate treatment on account of the higher probability of lymph node metastasis. A combination of local resection of primary tumor using EMR/ESD with subsequent D2 lymphadenectomy or laparoscopic lymph node dissection would be a suitable treatment choice. Moreover, patients with a high risk of lymph node metastasis may additionally need adjuvant radiotherapy and/or chemotherapy after initial curative therapy. Close follow-up is quite essential. In recent years, the evidence-based approach of sentinel node biopsy (SNB) is necessary and indispensible for stomach and function-preserving surgery and better quality of life for long-term survival (27). Therefore, SNB after initial endoscopic surgery is another less extensive surgery compared with D2 resection. Given the good prognosis of patients with early-stage gastric cancer without lymph node metastasis, SNB may be appropriated for patients who have early-stage gastric cancer which is undifferentiated, and/or with lymphovascular/submucosal invasion. If the result is positive for lymph node metastasis, the patient is committed to having a D2 resection. In contrast, the patient who has EGC but the primary tumor is differentiated mucosal invasion, and/or without lymphovascular invasion, is usually less likely to have lymph node metastasis, and for them endoscopic resection is an enough suitable treatment. In spite of the small single cohort, we spent a very long time observing the clinical biological behavior of EGC, and discovered the importance of lymph node metastasis in prognosis and recurrence/metastasis after surgery in EGC, which is helpful for future clinical practice.
In conclusion, the present study indicates that undifferentiated carcinoma and tumor with lymphovascular and submucosal invasion were associated with higher lymph node metastasis in EGC. D2 lymphadenectomy or laparoscopic lymph node dissection should be performed after endoscopic treatment in these patients. Moreover, patients with a poor prognosis and high recurrence/metastasis rate should have more rigorous follow-up or additional chemotherapy or radiation after D2 gastrectomy. Further investigations are needed to enlarge the cohort to a multicenter study in order to explain the differences in survival rates with different treatments.
Acknowledgements
This work was supported by Research on Strategy and Standard Project for Secondary Prevention of Gastrointestinal Tumor (No. 201202014) and National Key Technology R&D Program (Beijing Municipal Science and Technology Project Z121100007512010).
Disclosure: The authors declare no conflict of interest.
References
- Sim SH, Kim YJ, Oh DY, et al. The role of PET/CT in detection of gastric cancer recurrence. BMC Cancer 2009;9:73. [PubMed]
- Kwee RM, Kwee TC. Predicting lymph node status in early gastric cancer. Gastric Cancer 2008;11:134-48. [PubMed]
- Kitano S, Shiraishi N, Uyama I, et al. A multicenter study on oncologic outcome of laparoscopic gastrectomy for early cancer in Japan. Ann Surg 2007;245:68-72. [PubMed]
- Lee HH, Yoo HM, Song KY, et al. Risk of limited lymph node dissection in patients with clinically early gastric cancer: indications of extended lymph node dissection for early gastric cancer. Ann Surg Oncol 2013;20:3534-40. [PubMed]
- Ishikawa S, Togashi A, Inoue M, et al. Indications for EMR/ESD in cases of early gastric cancer: relationship between histological type, depth of wall invasion, and lymph node metastasis. Gastric Cancer 2007;10:35-8. [PubMed]
- Son SY, Park JY, Ryu KW, et al. The risk factors for lymph node metastasis in early gastric cancer patients who underwent endoscopic resection: is the minimal lymph node dissection applicable? A retrospective study. Surg Endosc 2013;27:3247-53. [PubMed]
- Yamashita K, Sakuramoto S, Shibata T, et al. Survival outcome of laparoscopic gastrectomy for clinical early (cT1) gastric cancer. Surg Today 2013;43:1013-8. [PubMed]
- Chen W, Zheng R, Zhang S, et al. Report of incidence and mortality in China cancer registries, 2009. Chin J Cancer Res 2013;25:10-21. [PubMed]
- Saka M, Katai H, Fukagawa T, et al. Recurrence in early gastric cancer with lymph node metastasis. Gastric Cancer 2008;11:214-8. [PubMed]
- Kim JW, Hwang I, Kim MJ, et al. Clinicopathological characteristics and predictive markers of early gastric cancer with recurrence. J Korean Med Sci 2009;24:1158-64. [PubMed]
- Youn HG, An JY, Choi MG, et al. Recurrence after curative resection of early gastric cancer. Ann Surg Oncol 2010;17:448-54. [PubMed]
- Park YD, Chung YJ, Chung HY, et al. Factors related to lymph node metastasis and the feasibility of endoscopic mucosal resection for treating poorly differentiated adenocarcinoma of the stomach. Endoscopy 2008;40:7-10. [PubMed]
- Okabayashi T, Kobayashi M, Nishimori I, et al. Clinicopathological features and medical management of early gastric cancer. Am J Surg 2008;195:229-32. [PubMed]
- Lee JH, Choi MG, Min BH, et al. Predictive factors for lymph node metastasis in patients with poorly differentiated early gastric cancer. Br J Surg 2012;99:1688-92. [PubMed]
- Kim JH, Kim HS, Seo WY, et al. External validation of nomogram for the prediction of recurrence after curative resection in early gastric cancer. Ann Oncol 2012;23:361-7. [PubMed]
- Lee JH, Choi IJ, Kook MC, et al. Risk factors for lymph node metastasis in patients with early gastric cancer and signet ring cell histology. Br J Surg 2010;97:732-6. [PubMed]
- Ryu KW, Choi IJ, Doh YW, et al. Surgical indication for non-curative endoscopic resection in early gastric cancer. Ann Surg Oncol 2007;14:3428-34. [PubMed]
- Kunisaki C, Takahashi M, Nagahori Y, et al. Risk factors for lymph node metastasis in histologically poorly differentiated type early gastric cancer. Endoscopy 2009;41:498-503. [PubMed]
- An JY, Baik YH, Choi MG, et al. Predictive factors for lymph node metastasis in early gastric cancer with submucosal invasion: analysis of a single institutional experience. Ann Surg 2007;246:749-53. [PubMed]
- Kunisaki C, Makino H, Takagawa R, et al. Tumor diameter as a prognostic factor in patients with gastric cancer. Ann Surg Oncol 2008;15:1959-67. [PubMed]
- Liu Y, Chen XH, Meng XH, et al. Multivariate prognostic study on node-positive gastric cancer: is tumor size a prognostic indicator? Hepatogastroenterology 2012;59:623-6. [PubMed]
- Lai JF, Kim S, Kim K, et al. Prediction of recurrence of early gastric cancer after curative resection. Ann Surg Oncol 2009;16:1896-902. [PubMed]
- Kikuchi S, Katada N, Sakuramoto S, et al. Survival after surgical treatment of early gastric cancer: surgical techniques and long-term survival. Langenbecks Arch Surg 2004;389:69-74. [PubMed]
- Zhuo W, Chen Y, Song XM, et al. Endostatin specifically targets both tumor blood vessels and lymphatic vessels. Front Med 2011;5:336-40. [PubMed]
- Gao P, Zhou GY, Zhang QH, et al. Clinicopathological significance of peritumoral lymphatic vessel density in gastric carcinoma. Cancer Lett 2008;263:223-30. [PubMed]
- Liang P, Hong JW, Ubukata H, et al. Increased density and diameter of lymphatic microvessels correlate with lymph node metastasis in early stage invasive colorectal carcinoma. Virchows Arch 2006;448:570-5. [PubMed]
- Ryu KW. The future of sentinel node oriented tailored approach in patients with early gastric cancer. J Gastric Cancer 2012;12:1-2. [PubMed]


