Inhibition of PI3K/Akt/mTOR signaling pathway enhances the sensitivity of the SKOV3/DDP ovarian cancer cell line to cisplatin in vitro
Introduction
As a serious and under-recognized tumor, ovarian cancer is the most lethal gynaecological malignancy and is the fourth most frequent cause of cancer-related deaths in women around the world. The high mortality is due to the fact that ovarian cancer often manifests with little or no specific symptoms at the early stage. The primary treatment for ovarian cancer is surgical resection of visible disease followed by adjuvant chemotherapy, which usually consists of a combination of platinum-based and taxane-based chemotherapy. More than 70% of such patients initially respond to cisplatin. However, the five-year survival rate is less than 25%. The high mortality rate is based on high proportion of advanced stage cases or aggressive cases experiencing chemoresistance and recurrence (1). For example, the 5-year survival rate of ovarian cancer cases at stage III and IV is about 28 and 16%, while the 5-year survival rate of stage I cases is up to 80% (2). Therefore, it is necessary to explore new approach to reverse chemotherapy resistance and to enhance sensitivity to platinum-based chemotherapy drugs.
The activation of PI3K/AKT/mTOR pathway can lead to an increase in cell proliferation, migration, invasion, and chemotherapy resistance of many cancers. For NSCLC patients, the aberrant activation of the PI3K/Akt/mTOR pathway is related to the resistance to gefitinib and a poor prognosis (3). For breast cancer, the activation of PI3K/AKT/mTOR pathway could increase the resistance of breast cancer cell clones to letrozole and develop an early resistance to letrozole in neo-adjuvant treatment for the patients (4). In ovarian cancer, the PI3K/AKT/mTOR pathway plays a key role in ovarian cancer tumorigenesis, progression, and chemotherapy resistance via the mutated or altered PI3K/AKT/mTOR pathway (5). In addition, the inhibition of the PI3K/Akt/mTOR pathway combined with classical anticancer agents is highly effective in several experimental studies (6), which could be the most appropriate approach for target therapy and to avoid acquired resistance in clinical.
This study aimed to evaluate the changes in cell cycle, apoptosis and the PI3K/AKT/mTOR pathway in ovarian carcinoma cells, SKOV3 and SKOV3/DDP, treated with PI-103 and cisplatin, and thus to explore the possible mechanism that PI-103 enhances the sensitivity of SKOV3/DDP ovarian cancer cell line to cisplatin.
Materials and methods
Cells and culture
Human ovarian cancer cells SKOV3 and the drug resistant human ovarian cancer cells SKOV3/DDP were obtained from Obstetrics and Gynecology Laboratory, Nanjing Medical University. RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) was used to culture cells. Cells were cultured at 37 °C in a humidified atmosphere with 5% CO2. Cells from exponentially growing cultures were used in all experiments.
Chemicals and antibodies
PI-103 were purchased from Cayman company (United States) and cisplatin (DDP) was purchased from Shandong Qilu Pharmaceutical Factory, which were dissolved in RPMI 1640 medium in the concentration of 1 g/L. CCK-8 kit, cell cycle and apoptosis detection kit were purchased from Beyotime biotechnology company (Jiangsu). FBS was purchased from Hangzhou Sijiqing Company, and RPMI 1640 medium was purchased from Gibco Company. Flow cytometry (FACS Vantage SE) was purchased from BD Company. Anti cyclin D1, anti p21 and anti-cleaved caspase-3 were purchased from CST Company. Anti Akt and anti Phospho-Akt (Ser473) were from Bioworld Company. Anti rps6 and anti Phospho-rps6 were from CST Company. Anti β-actin was purchased from Sigma Company.
The inhibition rates of SKOV3 and SKOV3/DDP treated with different concentration of PI-103
The growth inhibition rates of SKOV3 and SKOV3/DDP cells were assessed using the CCK8 assay. Briefly, the exponentially growing cells were digested, and single cell suspension was obtained. A total of 10,000 cells were plated in 96-well flat bottom plates. After 24 hours incubation, the culture media was replaced by fresh culture media containing PI-103 in concentrations of 0.022, 0.044, 0.088, 0.175, 0.35, 0.70 and 1.4 mg/L, and then continually cultured for 24, 48 and 72 h. CCK8 (20 μL/well) solution was added into each well and incubated for 2 h at 37 °C. After incubation, absorbance was determined by a microplate reader at 450 nm and absorbance values were expressed as the percentage of the untreated control. The test was repeated in triplicate. The growth inhibition rate of SKOV3 and SKOV3/DDP was calculated as follows:
Inhibition rate = (1– PI-103 group OD)/control group OD ×100%.
IC50 of DPP on SKOV3 and SKOV3/DDP
The IC50 of DPP for SKOV3 and SKOV3/DDP was assessed using the CCK8 assay. The cells were cultured in 96-well flat bottom plates as the same way described above, and the following concentration series of cisplatin was adopted, 0.78, 1.56, 3.13, 6.25, 12.50, 25.00, 50.00 and 100.00 mg/L. After 72 hours treatment, the inhibition rate was assessed using CCK8 assay, and IC50 was counted according to the concentration of cisplatin and the inhibition rate. The test was repeated in triplicate.
The effect of PI-103 on the IC50 of DPP for SKOV3 and SKOV3/DDP
The IC50s of cisplatin for SKOV3 and SKOV3/DDP treated with different concentrations of PI-103 were comparatively studied to evaluate the impact of PI-103 on the drug resistant. The cells were cultured in 96-well flat bottom plates as the same way described above, and then were treated with cisplatin and PI-103. The concentration series of cisplatin adopted were 0.78, 1.56, 3.13, 6.25, 12.50, 25.00, 50.00 and 100.00 mg/L and the concentration series of PI-103 were 0.088, 0.175, 0.35, 0.70 and 1.4 mg/L. After 72 hours treatment, the IC50s of cisplatin for SKOV3 and SKOV3/DDP in the different concentrations of PI-103 were calculated based on the CCK8 assay results.
The cell cycle and apoptosis of SKOV3 and SKOV3/DDP treated with certain concentration the PI-103 and DDP
Based on the IC50 results of DDP for SKOV3 and the inhibition rates of SKOV3/DDP cells treated with PI-103, the appropriate condition, DDP (3.13 mg/L) and PI-103 (0.35 mg/L), was selected to explore the cell cycle and apoptosis of SKOV3 and SKOV3/DDP. The two cell lines were treated as following: SKOV3, SKOV3 + cisplatin, SKOV3 + PI-103, SKOV3 + cisplatin + PI-103, SKOV3/DDP, SKOV3/DDP + cisplatin, SKOV3/DDP + PI-103 and SKOV3/DDP + cisplatin + PI-103. The cells were digested and single cell suspension was obtained after treated with cisplatin and PI-103 for 72 h, and 1 mL cell suspension (including 1×106 cells) was shifted to the 2 mL EP tubes. The cells were washed 3 times by pre-cooling PBS and then were fixed in pre-cooling 70% ethanol for 24 h. The tubes were centrifuged at 1,000 g for 3-5 minutes and the supernatant was removed carefully. The precipitation cells were washed by pre-cooling PBS again, and then the propidium (PI) staining solution was added into the tubes. The cells were tested by flow cytometry (488 nm) after incubation in 37 °C for 30 m in dark environment.
Western blot
Cells were treated as described above, and western blot was performed after 72 hours treatment. The cells were washed with ice-cold PBS, and lysis buffer were added to the cells with mixing and shocking on the ice, which made the cells fully cracked and total protein were extracted after centrifugation at 12,000 g for 20 minutes. The protein concentration was determined by the BCA kit. A 15 mg sample of protein was subjected to SDS-PAGE (12% gels). After electrophoresis, proteins of SDS-PAGE were transferred onto PDVF membranes in transfer buffer [25 mM Tris-HCl (pH 7.4) containing 192 mM glycine and 20% v/v methanol] using semidry transfer way (152 mA, 45 m). Membrane were blocked with 5% non-fat milk in Tris-buffered saline containing 0.1% Tween20 (TBST) at room temperature for 1 h and then incubated overnight at 4 °C with specific primary antibodies. After washing, the signals were detected with horseradish peroxidase-conjugated secondary antibody for 1 h, and visualized using the ECL chemiluminescent system (GE Healthcare, Munich, Germany) and exposed X-ray films. Relative optical densities and areas of bands were quantified using an image densitometer. The densitometric plots of the results were normalized to the intensity of the β-actin band.
Statistical analysis
Experimental data were presented as mean ± SEM. All statistical analysis was performed with use of the SPSS 13.5. t-test and one-way analysis of variance (ANOVA) followed by Tukey’s post hoc analysis was applied. A P value of <0.05 was considered as statistically significant.
Results
The inhibition rates of SKOV3 and SKOV3/DDP cells treated with different concentration of PI-103
In order to observe the impact of PI-103 on the cell proliferation of SKOV3 and SKOV3/DDP, the inhibition rates of SKOV3 and SKOV3/DDP cells treated with different concentration of PI-103 at different time were calculated. As shown in Figure 1, the growth inhibition rate increases along with incubation time. PI-103 increases the inhibition rate of SKOV3 and SKOV3/DDP cells in a dose dependent manner at the three studied time points, i.e., 24, 48 and 72 h. The inhibition rates of SKOV3 and SKOV3/DDP cells were markedly increased when the PI-103 concentration >0.35 mg/L. Treated with PI-103 0.35 mg/L, the inhibition rates of SKOV3 cells were 17.79%, 28.24% and 32.68% at 24, 48 and 72 h, and those of SKOV3/DDP cells were 15.82%, 25.48% and 32.53% (Figure 1). There was no significant difference considering the inhabitation rate between SKOV3 and SKOV3/DDP cells at each time point (P>0.05). The results revealed no difference considering the cytotoxicity of the PI-103 on SKOV3 and SKOV3/DDP cells.

The IC50 of cisplatin for SKOV3 and SKOV3/DDP cells
The inhibition rates of SKOV3 and SKOV3/DDP cells treated with different concentrations of cisplatin were determined to calculate the IC50. The results revealed that SKOV3 cells were very sensitive to cisplatin and almost all cells died treated with cisplatin 100 mg/L. However, SKOV3/DDP cells had stronger resistance to cisplatin and the inhibition rate was 85.25% treated with cisplatin 100 mg/L. The IC50s of DDP for SKOV3 and SKOV3/DDP cells were 3.31 and 13.96 mg/L, respectively (Figure 2).
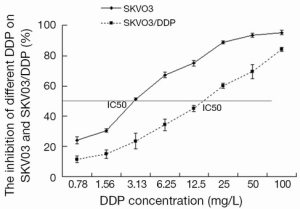
The impact of PI-103 on the IC50 of cisplatin for SKOV3 and SKOV3/DDP cells
To explore whether the combination of PI-103 and cisplatin could exert the more inhibitive effects on SKOV3/DDP, SKOV3 and SKOV3/DDP cells were treated with indicated concentrations of PI-103 and cisplatin, and the optical density (OD) was measured to calculate the cell growth inhibition rate and IC50. The inhibition rates of cisplatin for SKOV3 and SKOV3/DDP treated with the different concentration of PI-103 were displayed in (Figure 3A,B). For PI-103 0 mg/L, the IC50s of cisplatin for SKOV3 and SKOV3/DDP cells were 3.13 and 13.95 mg/L. The IC50s of cisplatin for SKOV3 and SKOV3/DDP cells were changed to 1.12 and 3.85 mg/L treated with PI-103 1.4 mg/L (Figure 3C,D). The results showed that the PI-103 could significantly increase the sensitive of SKOV3/DDP cells to cisplatin.
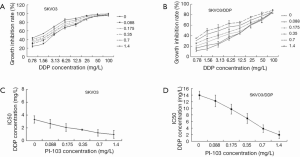
The cell cycle and apoptosis of SKOV3 and SKOV3/DDP cells treated with certain concentration of PI-103 and DDP
PI-103 and DDP blocks cell cycle progress in SKOV3 and SKOV3/DDP
In this work, DDP (3.13 mg/L) and PI-103 (0.35 mg/L) was applied to study the effects of DDP and PI-103 on cell cycle and apoptosis of SKOV3 and SKOV3/DDP.
The results were shown in Figure 4A,B. The G0/G1 DNA content of SKOV3 cells was significantly higher than that of SKOV3/DDP cells (38.00±1.32 vs. 29.21±3.56, P<0.05), and treated with cisplatin for 72 h, the G0/G1 DNA content was significantly changed and G0/G1 DNA content of SKVO cells was significantly more than that of SKOV3/DDP (55.20±6.85 vs. 31.88±2.39, P<0.05). This suggests that the resistance of SKOV3/DDP may be correlated with decreased DNA content of G0/G1. After treated with PI-103 for 72 h, the G0/G1 DNA content was not significant changed (51.77±4.00 for SKOV3 vs. 52.18±2.80 for SKOV3/DDP, P>0.05), the G0/G1 DNA content was significantly increased compared to not treated (51.77±4.00 for SKOV3 and 52.18±2.80 for SKOV3/DDP vs. 38.00±1.32 for SKOV3 and 29.21±3.56 for SKOV3/DDP, P<0.05). In the combination treatment, cisplatin + PI-103 could significantly increase the content of G0/G1 in SKOV3 and SKOV3/DDP cells comparing to treated with cisplatin and PI-103 alone (P<0.05) (Figure 4A,B). The results suggested that the combination could significantly increase the G0/G1 DNA content of SKOV3 and SKOV3/DDP cells.
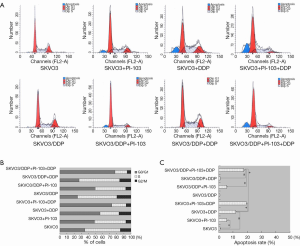
Apoptosis of SKOV3 and SKOV3/DDP cells treated with PI-103 and DDP
In addition to the cell cycle, the cell apoptosis was also tested by flow cytometry, and the results were shown in Figure 4A,C. Fewer apoptosis cells were detected in the SKOV3 group, SKOV3/DDP group and SKOV3/DDP + cisplatin group, and the G0/G1 DNA contents were also relatively less. However, in the SKOV3 + cisplatin, SKOV3 + PI-103 and SKOV3/DDP + PI-103 groups, the apoptosis rates were significantly increased compared with the SKOV3, SKOV3/DDP and SKOV3/DDP + cisplatin group (11.84%, 5.51%, 19.37 vs. 0.85%, 0.71%, 0.35%, P<0.01). The combination of DDP + PI-103 induced more apoptosis in SKOV3 and SKOV3/DDP cells (19.37%, 17.49% vs. 11.84%, 5.51%, 5.55%, P<0.01) (Figure 4A,C).
The expression of cyclin D1, p21, cl-caspase-3
Next, the G1/S-specific protein cyclin-D1, the potent cyclin-dependent kinase inhibitor (CKI) p21 and activated apoptosis protein cleaved caspase-3 were investigated by Western blot (Figure 5A). The expression of cyclin-D1 was displayed in Figure 5B. The expression of cyclin-D1 was increased in the SKOV3, SKOV3/DDP and SKOV3/DDP + cisplatin groups compared with the other groups (*P<0.05, #P<0.01). PI-103 and cisplatin could significantly decreased the cyclin-D1 protein in SKOV3 cells and the combination induced more effects (*P<0.05, #P<0.01). Though SKOV3/DDP was not sensitive to cisplatin, the cyclin-D1 expression was also significantly decreased (#P<0.01), which was no significantly different compared with the SKOV3 + PI-103 + cisplatin group.
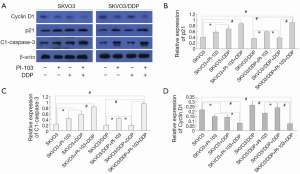
The expression of cyclin-dependent kinase inhibitor (CKI) p21 was displayed in Figure 5C. PI-103 significantly increased the expression of p21 in SKOV3 and SKOV3/DDP cells, and DDP did as well in SKOV3 cells. The combination of PI-103 + DDP increased the p21 expression significantly in the SKOV3 and SKOV3/DDP cells (*P<0.05, #P<0.01).
The expression clease-caspase-3, the activated apoptosis protein, was also detected. The activated caspase-3 was significantly increased in the treatment group except the SKOV3/DDP + cisplatin group, and the combination showed better effects on activating caspase-3 in SKOV3 and SKOV3/DDP + DDP cells (*P<0.05, #P<0.01) (Figure 5C).
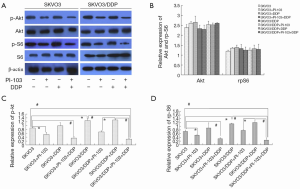
Phosphorylation of Akt and rpS6
The expressions of phospho-Akt and phospho-S6 in SKOV3 and SKOV3/DDP cells were also examined. The results were shown in Figure 6A,B. The expression of p-Akt was higher in SKOV3/DDP cells than in SKOV3 cells (*P<0.05), and PI-103 could significantly decrease the p-Akt expression, while cisplatin could not. The combination of PI-103 + cisplatin further decreased the expression of p-Akt (*P<0.05, #P<0.01). The expression of p-rpS6 was displayed in Figure 6A,C, and similar trend as p-Akt was observed. There were no significant changes in the total protein of Akt and rpS6 Figure 6A,D.
Discussion
Chemotherapy is one of the important therapeutic strategies for ovarian cancer, and platinum-based combination chemotherapy is most widely applied in clinical practice. However, the clinical outcome is often not satisfactory due to drug resistance. Cisplatin is not recommended for cases developed drug resistance. Therefore, it is currently an urgent problem to solve platinum resistance and improve the chemotherapy sensitivity to platinum-based chemotherapy drugs for the treatment of ovarian cancer.
The drug resistance of ovarian cancer to chemotherapy is the results of the complex interaction between multi-gene and multiple factors (7). Previous studies showed that the multidrug resistance gene 1/P-glycoprotein (MDR1/P-gp), the hypoxic microenvironment of ovarian cancer (8) as well as abnormal regulation of apoptosis could induce drug resistance. PI3K/Akt/mTOR pathway is involved in the cancer development, and its aberrant activation may induce malignant transformation and chemoresistance (9,10). Fraser et al. found that the activated Akt protein could induce the ovarian cancer drug resistance through negative regulation of P53 (11). P13K inhibitor could improve the ovarian cancer sensitivity to cisplatin by increasing the mitochondrial Bax translocation and cytC release (12). Some studies also demonstrated the correlation of phosphorylation of mTOR with drug resistance. Mabuchi et al. reported that the mTOR phosphorylation levels was higher in cisplatin-resistant ovarian cancer cell line than in cisplatin-sensitive cell lines, and the former is more sensitive to the mTOR inhibitor RAD001 (13), which suggests that the higher mTOR phosphorylation is involved in cisplatin resistance. The activation of the Akt/mTOR pathway can inhibit cisplatin-induced apoptosis of ovarian cancer cells, causing cisplatin resistance in ovarian cancer cells (14).
In this work, we firstly explore the effect of PI-103 on the proliferation of SKOV3 and SKOV3/DDP cells, and the results showed that there was no obvious difference between the two cells. Based on the proliferation curve, 0.35 mg/L was selected as the subsequent concentration. In addition, we found that the IC50 of cisplatin for SKOV3 and SKOV3/DDP cells was 3.31 and 13.96 mg/L, which revealed the strong drug resistance of SKOV3/DDP cells to cisplatin. In the next step, the combination of PI-103 + cisplatin was introduced to explore the inhibition effect. The results showed that the PI-103 could significantly increase the sensitivity of SKOV3/DDP to cisplatin.
Previous studies had demonstrated an association between cell cycle regulation and tumor development, and blockage of the cell cycle progression has become an appropriate target therapy (15,16). Our results indicated that PI-103 could significantly increase the G0/G1 DNA content in SKOV3 and SKOV3/DDP cells and the apoptosis rate treated with cisplatin. The expression levels of cell cyclic proteins and apoptosis proteins were also changed. This suggests that PI-103 could reduce drug resistance of ovarian cancer cell SKOV3/DDP through inhibiting cell proliferation and inducing apoptosis, and PI-103 could enhance the sensitivity of SKOV3/DDP to the cisplatin. PI-103 may be an emerging paradigm for the treatment of cisplatin-resistant ovarian cancer cases.
Treated with PI-103 and cisplatin, the cell cycle was changed. Hence, a question is that whether the proteins in the PI3K/Akt/mTOR signal pathway were also changed. Our results indicated that PI-103 could inhibit the activated Akt and rpS6 but cisplatin could not. However, the combination of PI-103 and cisplatin showed stronger ability of inhibiting the activated Akt and rpS6 either in SKOV3 or SKOV3/DDP cells. This suggests that the interaction between PI-103 and cisplatin could increase the ability of inhibiting activated Akt and rpS6 and inhibit the PI3K/Akt/mTOR signal pathway, which is a very important signaling pathway in apoptosis and ovarian cancer (17,18). Schwab et al. reported that PI-103 inhibits the chordoma cell proliferation and promots apoptosis (19). Chen et al. found that PI-103 could significantly increase the sensitivity of malignant glioma to radiotherapy (20). Many in vitro studies showed that PI-103 also improves the anti-tumor effect of some chemotherapy drugs such as cisplatin, paclitaxel, vincristine, doxorubicin (21).
Tumor cells could dynamically adapt to chemotherapy drugs through complex signaling networks, and this results in drug resistance. Blocking one of such pathways might increase the sensitivity of tumor cells to chemotherapy. Nowadays, it is becoming increasingly apparent that the effects of mostly applied target therapies are limited due to drug resistance. Our results revealed that the dual inhibitor PI-103 could enhance the sensitivity of SKOV3/DDP ovarian cancer cell line to cisplatin, which maybe through inhibiting the activated PI3K/Akt/mTOR signal pathway and increasing the G0/G1 DNA content. Based on this, PI-103 could be the potential candidate to solve drug resistance for ovarian cancer cases. Our results suggest that concomitant inhibition of PI3K and mTOR could be a promising therapeutic strategy for the treatment of ovarian cancer cases developed—drug resistant to cisplatin. However, the detailed and accurate mechanism still needs to be studied in future.
Conclusions
In this research, we explore possible mechanism of enhancing the sensitivity of SKOV3/DDP ovarian cancer cell line to cisplatin chemotherapy by treating with PI-103, a dual inhibitor of phosphatidylinositide 3-kinase and mTOR. The results showed that PI-103 could significantly increase the sensitivity of SKVO3/DDP cells to cisplatin through inhibiting the activation of PI3K/Akt/mTOR signaling pathway and the dual inhibit effect could be a promising therapeutic strategy for the treatment of ovarian cancer cases developed—drug resistant to cisplatin.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (30973475), the Natural Science Foundation of Jiangsu Province (BK2012749) and the Maternal and Child Health Research Project of Jiangsu Province (F201351).
Disclosure: The authors declare no conflict of interest.
References
- Omura GA, Brady MF, Homesley HD, et al. Long-term follow-up and prognostic factor analysis in advanced ovarian carcinoma: the Gynecologic Oncology Group experience. J Clin Oncol 1991;9:1138-50. [PubMed]
- Conte PF, Cianci C, Gadducci A. Up date in the management of advanced ovarian carcinoma. Crit Rev Oncol Hematol 1999;32:49-58. [PubMed]
- Janmaat ML, Kruyt FA, Rodriguez JA, et al. Response to epidermal growth factor receptor inhibitors in non-small cell lung cancer cells: limited antiproliferative effects and absence of apoptosis associated with persistent activity of extracellular signal-regulated kinase or Akt kinase pathways. Clin Cancer Res 2003;9:2316-26. [PubMed]
- Cavazzoni A, Bonelli MA, Fumarola C, et al. Overcoming acquired resistance to letrozole by targeting the PI3K/AKT/mTOR pathway in breast cancer cell clones. Cancer Lett 2012;323:77-87. [PubMed]
- Dobbin ZC, Landen CN. The Importance of the PI3K/AKT/MTOR Pathway in the Progression of Ovarian Cancer. Int J Mol Sci 2013;14:8213-27. [PubMed]
- Mabuchi S, Altomare DA, Cheung M, et al. RAD001 inhibits human ovarian cancer cell proliferation, enhances cisplatin-induced apoptosis, and prolongs survival in an ovarian cancer model. Clin Cancer Res 2007;13:4261-70. [PubMed]
- Perez RP, Hamilton TC, Ozols RF, et al. Mechanisms and modulation of resistance to chemotherapy in ovarian cancer. Cancer 1993;71:1571-80. [PubMed]
- Wartenberg M, Ling FC, Müschen M, et al. Regulation of the multidrug resistance transporter P-glycoprotein in multicellular tumor spheroids by hypoxia-inducible factor (HIF-1) and reactive oxygen species. FASEB J 2003;17:503-5. [PubMed]
- Haluska FG, Tsao H, Wu H, et al. Genetic alterations in signaling pathways in melanoma. Clin Cancer Res 2006;12:2301s-2307s. [PubMed]
- Nicholson KM, Anderson NG. The protein kinase B/Akt signalling pathway in human malignancy. Cell Signal 2002;14:381-95. [PubMed]
- Fraser M, Leung BM, Yan X, et al. p53 is a determinant of X-linked inhibitor of apoptosis protein/Akt-mediated chemoresistance in human ovarian cancer cells. Cancer Res 2003;63:7081-8. [PubMed]
- Lee S, Choi EJ, Jin C, et al. Activation of PI3K/Akt pathway by PTEN reduction and PIK3CA mRNA amplification contributes to cisplatin resistance in an ovarian cancer cell line. Gynecol Oncol 2005;97:26-34. [PubMed]
- Mabuchi S, Kawase C, Altomare DA, et al. mTOR is a promising therapeutic target both in cisplatin-sensitive and cisplatin-resistant clear cell carcinoma of the ovary. Clin Cancer Res 2009;15:5404-13. [PubMed]
- Peng DJ, Wang J, Zhou JY, et al. Role of the Akt/mTOR survival pathway in cisplatin resistance in ovarian cancer cells. Biochem Biophys Res Commun 2010;394:600-5. [PubMed]
- Shukla S, Gupta S. Molecular mechanisms for apigenin-induced cell-cycle arrest and apoptosis of hormone refractory human prostate carcinoma DU145 cells. Mol Carcinog 2004;39:114-26. [PubMed]
- Singh RP, Agarwal C, Agarwal R. Inositol hexaphosphate inhibits growth, and induces G1 arrest and apoptotic death of prostate carcinoma DU145 cells: modulation of CDKI-CDK-cyclin and pRb-related protein-E2F complexes. Carcinogenesis 2003;24:555-63. [PubMed]
- Morgensztern D, McLeod HL. PI3K/Akt/mTOR pathway as a target for cancer therapy. Anticancer Drugs 2005;16:797-803. [PubMed]
- Cortot A, Armand JP, Soria JC. PI3K-AKT-mTOR pathway inhibitors. Bull Cancer 2006;93:19-26. [PubMed]
- Schwab J, Antonescu C, Boland P, et al. Combination of PI3K/mTOR inhibition demonstrates efficacy in human chordoma. Anticancer Res 2009;29:1867-71. [PubMed]
- Chen JS, Zhou LJ, Entin-Meer M, et al. Characterization of structurally distinct, isoform-selective phosphoinositide 3'-kinase inhibitors in combination with radiation in the treatment of glioblastoma. Mol Cancer Ther 2008;7:841-50. [PubMed]
- Bender A, Opel D, Naumann I, et al. PI3K inhibitors prime neuroblastoma cells for chemotherapy by shifting the balance towards pro-apoptotic Bcl-2 proteins and enhanced mitochondrial apoptosis. Oncogene 2011;30:494-503. [PubMed]


