Dermatosis as the initial presentation of gastric cancer: two cases
Introduce
Paraneoplastic dermatoses are certain skin conditions associated with tumor, such as acanthosis nigricans, dermatomyositis, erythroderma, ichthyosis and so on. The tumors ichthyosis associated with are mostly adenocarcinoma, especially gastrointestinal tumors, accounting for 90%. The tumors dermatomyositis related to are breast cancer, uterine cancer, ovarian cancer, gastric cancer, lung cancer, malignant lymphoma, liver cancer, prostate cancer, kidney cancer, leukemia, and so on. The tumors associated with erythroderma are T-cell lymphoma, lung cancer, stomach cancer, and so on (1-15). This paper described one case of dermatomyositis and erythroderma accompanying gastric cancer respectively.
Case reports
Case 1
A 57-year-old man was admitted to Rheumatism Immunity Branch of The First Hospital of Nanjing with skin erythema and muscle soreness for half a month on May 4th, 2012. Erythema of the inferior orbital, both sides of the nose, forehead, and neck made a surprise attack on the patient half a month ago. Later, aching pain of double shoulder joints, double upper arm, and double side thigh, and movement disturbance of four limbs gradually appeared. So, he went to The First Hospital of Nanjing for further cure.
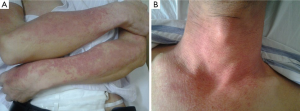
Physical examination: red papules were scattered in his frontal part, double eyelid, orbit below, neck, and limbs without skin mucous membrane burst, and superficial lymph node enlargement (Figure 1).
Laboratory examination: blood routine test showed that white blood cell count was 5.8×109/L (normal range, 4×109-10×109/L); the proportion of neutrophile granulocyte was 77% (normal range, 51-75%). Erythrocyte sedimentation rate (ESR): 8 mm/h (normal range, 0-15 mm/h). Rheumatism three items and immune globulin showed a normal level in serum, but complement C4 (153.00 mg/L) was low-grade. The test of autoantibody suggested 1:1,000 positive of antinuclear antibody of ANA.
His manifestation of skin, and the laboratory data fulfilled the criteria for the diagnosis of dermatomyositis. As the patient had the medical history of “gastric ulcer with bleeding”, stomachoscopy was made to show lesion in the preventriculus, which was proved to be poorly differentiated carcinoma by pathological examination. Then the patient was transferred to department of gastrointestinal surgery of our hospital for operation.
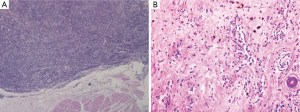
Radical gastrectomy was performed with general anesthesia on May 13th 2012. Firstly a small part of skin, and muscle was biopsied. Radical total gastrectomy was performed with lymphadenectomy (D2) and esophagojejunostomy of Roux-en-Y. On the second day after the operation, the aching pain of muscle relieved obviously, the erythema also receded a little. The postoperative pathological report showed the poorly differentiated ulcerative adenocarcinoma in the anterior wall of the stomach around the cardia with local neuroendocrine differentiation IB (T2, N0, cM0). The following proteins were expressed in cancer cells: EGFR (–), TS (–), β-tubulin (+++), ERCC1 (–), BRCA1 (–), CerbB2 (–), VEGF (+), COX2 (++), Syn focal (+), CgA (–), P63 (+), Ki67 (30%+). The skin biopsy showed a few of neutrophile granulocytes infiltrated into the superficial layer of dermis and cutaneous appendages. The muscle biopsy showed perivascular neutrophile granulocyte cells infiltrated into muscle bundles with a small amount of muscle fiber degeneration and necrosis. The epidermis and muscle were related with dermatomyositis. After one month, the patient still felt muscle aching pain with skin erythema. He visited Department of Rheumatism Immunity Branch for further examination and be treated with oral prednisone (40 mg QD). In the last six months, the patient’s symptoms improved a lot, but still exist.
Case 2
The patient, a 66-year-old man, was hospitalized in Department of Endocrinology in Drum Tower Hospital due to hypoglycemia on August 17th, 2010. He had a history of type 1 of diabetes mellitus for about forty years with insulin therapy. The hypoglycemia often occurred. Last year the patient complained of red papules on his back without obvious precipitating factors. Gradually the similar red papules spread from the head and neck to the trunk limbs, with severe itching. He was treated as chronic eczema, dermatitis or some other skin diseases with oral antihistamines and dexamethasone. He felt a little relief. However, the symptoms got worse with obvious desquamation and itching four months ago. Furthermore his hair lost gradually.
Physical examination: red papules were scattered in his head, face, trunk and limbs. And the papules partially mixed together with swelling, desquamation, itching and also tenderness (Figure 2). Several lymph nodes (0.5-3 cm) were touched in bilateral armpits, with clear border and evident tenderness.

Laboratory examination: blood routine test showed that eosinophil count was 1.7×109/L, the proportion of eosinophils 13.7% (normal range, 0.5-5%), monocyte count 1.1×109/L, the proportion of monocytes 9.1% (normal range, 3-8%), lymphocyte count 0.5×109/L, the proportion of lymphocyte 4.2% (normal range, 20-40%), hemoglobin 82 g/L (normal range, 131-172 g/L). IgG (18.5 g/L) and IgA (4.52 g/L) showed a high level in serum, but complement C4 (0.16 g/L) was low-grade. Erythrocyte sedimentation rate was 60 mm/h (normal range, 0-15 mm/h). The chest X-ray showed the change of interstitial pneumonia. Color Doppler ultrasound of liver showed enlargement of liver’s volume and splenomegaly. The test of autoantibody suggested 1:100 positive of antinuclear antibody of HEP2/granular pattern of monky’s liver.
In consideration of the manifestation of the patient’s body skins and the laboratory data, the endocrinologists asked the dermatologists and the rheumatologists for consultation. Erythroderma was considered according to the medical history, physical examination, laboratory data, and the treatment of antihistamines to control symptoms. Meanwhile, tumor markers were detected to rule out the tumors, which may induce erythroderma. And the levels of carcino-embryonic antigen (CEA) (normal range, 0-10 ng/mL), carbohydrate antigen (CA) 125 (normal range, 0-5 ng/mL), and CA50 (normal range, 0-21 ng/mL) were 15.10 ng/mL, 15.10 ng/mL, and 22.10 U/mL respectively, while alpha fetoprotein (AFP), CA72-4, and CA19-9 were normal. Furthermore, an ulcerative mass was detected in the place of the cardia under endoscopy and barium meal examination, which was confirmed as adenocarcinoma. Position emission tomography-computed tomography (PET-CT) imaging showed that one irregular mass in cardia was of hypermetabolism [standard uptake value (SUV) max 3.2, delay imaging SUVmax 5.9], and multiple small lymph nodes in bilateral armpits were not of hypermetabolism. Then the patient was transferred to Department of Gastrointestinal Surgery for operation.
Radical gastrectomy was performed with general anesthesia on Sep 14th 2010. Firstly a small part of skin and muscle was biopsied. Radical total gastrectomy was performed with lymphadenectomy (D2) and esophagojejunostomy of Roux-en-Y. On the first day after the operation, the pruritus relieved obviously, the erythema also receded a little, and the eosinophilic cell could not be detected. The postoperative pathological report showed the poorly differentiated ulcerative adenocarcinoma in the posterior wall of the stomach around the cardia (diffusing type of the classification of Lauren) (IIB, T3, N1, cM0). The following proteins were expressed in cancer cells: EGFR (+), P53 (70%+), COX2 (++), CerbB2 (+), VEGF (++), Ki67 (40%+), SSTR2 (+), Syn (–), CgA (–), CD56 focal (+). The skin biopsy showed perivascular neutrophile granulocyte cell infiltrate into skin biopsy with a few of leukomonocyte in the dermis. The epidermis was related erythroderma (Figure 3).
After one month, the symptoms of pruritus and desquamation improved a lot, the erythema and the papule also receded. Eosinophil count decreased to 0.2×109/L (2.0%). Unfortunately, the patient felt itching severely again about three months after the operation and he visited Department of Dermatology for further examination. The biopsy of the skin on right upper extremity showed accentuation of squamous epithelium’s keratinization, some eosinophils cells infiltrate in the dermis. Then, the patient was given by some symptomatic and supportive therapies. In the last six months, the symptoms of pruritus and desquamation improve a lot, but still exist.
Discussion
Paraneoplastic dermatoses are known to be certain dermatosis related with tumor, and the symptoms were parallel with the course of tumor (16). Research showed that etiology was associated with active factors or hormone secreted by tumor, but the specific pathogenesis is not very clear. The common paraneoplastic dermatoses are acanthosis nigricans, acquired ichthyosis, dermatomyositis, erythroderma, and so on.
Dermatomyositis is a kind of autoimmune disease with dermatitis and myositis, which is individualism or overlap exists with systemic lupus erythematosus (SLE), scleroderma, rheumatoid disease, and so on. The clinical manifestations are dermatitis, myositis, and constitutional symptoms such as high fever and joint pain. The pathogenic factor is related with infection of oxoplas or virus, and tumor is also a factor. At present, the malignancy correlated with dermatomyositis is breast cancer, ovarian cancer, ovarian cancer, gastric cancer, lung cancer, malignant lymphoma, mediastinum cancer, liver cancer, prostate cancer, kidney cancer, leukemia, and so on (1-15). Up to now, seven cases of gastric cancer were reported to be correlated with dermatomyositis (Table 1). Dermatomyositis accompanying malignant tumor is common in people between 40-60 years, and the incidence was 15-50% (22). The biggest risk of dermatomyositis is associated with malignant tumor and the older, the higher opportunity of tumor accompanying (23). Callen reported that tumor incidence was 71% in over 50-year-old patients with dermatomyositis (24). Our patient, a 57-year-old man, appeared erythema of neck and face suddenly and felt muscular soreness later. The laboratory examination showed that creatine kinase and lactate dehydrogenase (LDH) rised. In consideration of the manifestation of the patient’s body skins and the laboratory data, the diagnosis of dermatomyositis was made. The patient had the medical history of “gastric ulcer with bleeding”, so the stomachoscopy examination was made to discover lesion. This case suggests that, for sudden dermatomyositis patient, system checks should be made to eliminate tumor in conjunction with the past history.
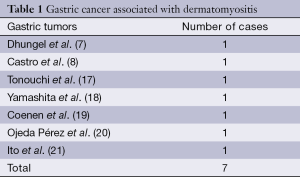
Full table
Papuloerythroderma, also called exfoliate dermatitis, was firstly reported by Ofuji in 1984 (25). Its, typical manifestations are diffuse flushing, papules, infiltration, swelling and desquamation in almost all our body, especially in the face and the creases (the “deck-chair” sign) (26). Besides the skins, it may also invade the mucous, the skin appendages, the lymph node, even and the viscera. The mucosal invasion may usually manifest as conjunctivitis, blear-eye, keratitis, corneal or mouth ulcers, or the mucosal erosion of women’s vagina, urethra or anus. Peripheral eosinophilia, lymphopenia, and elevated serum immunoglobulin (Ig) E levels occur in some cases (27). Erythroderma can be induced by lots of factors, such as psoriasis, eczema, seborrheic dermatitis, pityriasis rubra pilaris, lichen planus or some drugs [aspirin (28) and furosemid (29)]. Furthermore, erythroderma is related with malignant lymphoma (30), especially cutaneus T-celllymphoma (31), leukemi, mycosis (32), fungoides (33) and many other diseases, even the common gallstones, fungous infections of the skins, excessive eosinophilia syndrome, dermatomyositis (34), acquired immune deficiency syndrome (35), or hepatitis C virus infection.
There are some reports, which support the opinion that visceral tumors such as esophagus, stomach, colon, gallbladder, lung, kidney, prostate, nasopharynx, or pericardium are closely related with erythroderma (36-38). The reports on papuloerythroderma with visceral tumors were summarized in Table 2. Here we describe a case report, which confirmed this relationship between gastric cancer and erythroderma further. In addition, the patient had interstitial pneumonia simultaneously. Therefore, it was concluded that erythroderma and interstitial pneumonia are both a kind of paraneoplastic syndromes, which is firstly reported. This case with no primary skin disease, appeared red papules under no obvious predisposing causes, got no effect after symptomatic treatment, and proved to be associated with gastric cancer by stomachoscopy. As a result, some elderly patients with no primary skin disease, suffering from erythroderma and getting no curative effect through a variety of active treatment, should be highly suspected to accompany tumor.

Full table
Conclusions
Until now, there is not exact mechanism on the relationship between gastric cancer and paraneoplastic dermatoses, which is worth investigating further. Paraneoplastic dermatoses can be seen as the early manifestation of visceral carcinomas. As a result, visceral tumors should be excluded in the patients with paraneoplastic dermatoses. Tumors can be detected early with biomakers, gastrointestinal endoscope, and even PET/CT.
Acknowledgements
Consent: Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consents is available for review by the Editor-in-Chief of this journal.
We thank the patients for giving us written consent for publishing their details.
Disclosure: The authors declare no conflict of interest.
References
- Pectasides D, Koumpou M, Gaglia A, et al. Dermatomyositis associated with breast cancer. Anticancer Res 2006;26:2329-31. [PubMed]
- Celebi S, Gül A, Kamali S, et al. Amyopathic dermatomyositis associated with cervical cancer. Clin Rheumatol 2001;20:438-40. [PubMed]
- Chao LW, Wei LH. Dermatomyositis as the initial presentation of ovarian cancer. Taiwan J Obstet Gynecol 2009;48:178-80. [PubMed]
- Scheinfeld NS. Ulcerative paraneoplastic dermatomyositis secondary to metastatic breast cancer. Skinmed 2006;5:94-6. [PubMed]
- Nakamura S, Umeda T, Yokozeki H, et al. Possible involvement of tumour-derived interleukin-6 in dermatomyositis associated with cervical squamous cell carcinoma. Br J Dermatol 1997;136:475-6. [PubMed]
- Valverde R, Sánchez-Caminero MP, Calzado L, et al. Dermatomyositis and punctate porokeratotic keratoderma as paraneoplastic syndrome of ovarian carcinoma. Actas Dermosifiliogr 2007;98:358-60. [PubMed]
- Dhungel S, Chalise SN, Kandel S, et al. Dysphagia as a presenting symptom in a case of dermatomyositis and occult gastric malignancy. Nepal Med Coll J 2005;7:77-8. [PubMed]
- Castro C, Khan Y, Awasum M, et al. Case report: primary gastric melanoma in a patient with dermatomyositis. Am J Med Sci 2008;336:282-4. [PubMed]
- Przybylski G, Jarzemska A, Czerniak J, et al. A case report of a patient with dermatomyositis as a prodromal sign of lung cancer. Pol Arch Med Wewn 2008;118:143-7. [PubMed]
- Park JK, Subhawong AP, Ziminski CM, et al. Dermatomyositis as the initial presentation of a large anaplastic T-cell lymphoma. J Clin Oncol 2011;29:e378-80. [PubMed]
- Shrivastav A, Kumar V, Pal J. Dermatomyositis associated with acute myelocytic leukemia. Rheumatol Int 2010;30:671-3. [PubMed]
- Mooney CJ, Dunphy EJ, Stone B, et al. Identification of autoantibodies elicited in a patient with prostate cancer presenting as dermatomyositis. Int J Urol 2006;13:211-7. [PubMed]
- Hassoun N, Agabrya A, Levy Y. Mediastinal cancer presenting as dermatomyositis--a case report. Harefuah 2011;150:574-7, 618, 617.
- Kee SJ, Kim TJ, Lee SJ, et al. Dermatomyositis associated with hepatitis B virus-related hepatocellular carcinoma. Rheumatol Int 2009;29:595-9. [PubMed]
- Shinohara N, Harabayashi T, Suzuki S, et al. Advanced renal pelvic carcinoma associated with dermatomyositis. Int J Urol 2005;12:906-8. [PubMed]
- Schimrigch K, Blaes F. Paraneoplastic syndromes. Zh Nevrol Psikhiatr Im S S Korsakova 2002;102:48-9. [PubMed]
- Tonouchi H, Miki C, Masato K. Gastric cancer associated with dermatomyositis accompanied by photoallergy. Gan To Kagaku Ryoho 2001;28:689-91. [PubMed]
- Yamashita K, Hosokawa M, Hirohashi S, et al. Epstein-Barr virus-associated gastric cancer in a patient with dermatomyositis. Intern Med 2001;40:96-9. [PubMed]
- Coenen C, Wedmann B, Bauer KH, et al. Dermatomyositis as a paraneoplastic syndrome. A case report. Fortschr Med 1989;107:720-2. [PubMed]
- Ojeda Pérez E, Garmendia Tolosa MT, Sellares Ribera R, et al. Polyradiculoneuritis-dermatomyositosis as a paraneoplastic syndrome in stomach cancer. Rev Clin Esp 1983;169:269-70. [PubMed]
- Ito K, Imafuku S, Hamaguchi Y, et al. Case report of anti-transcription intermediary factor-1-γ/α antibody-positive dermatomyositis associated with gastric cancer and immunoglobulin G4-positive pulmonary inflammatory pseudotumor. J Dermatol 2013;40:567-9. [PubMed]
- Callen JP. The value of malignancy evaluation in patients with dermatomyositis. J Am Acad Dermatol 1982;6:253-9. [PubMed]
- Raffel GD, Gravallese EM, Schwab P, et al. Diagnostic dilemmas in oncology: case 2. Dermatomyositis and ovarian cancer. J Clin Oncol 2001;19:4341-3. [PubMed]
- Callen JP. Dermatomyositis and female malignancy. J Surg Oncol 1986;32:121-4. [PubMed]
- Ofuji S, Furukawa F, Miyachi Y, et al. Papuloerythroderma. Dermatologica 1984;169:125-30. [PubMed]
- Farthing CF, Staughton RC, Harper JI, et al. Papuloerythroderma--a further case with the ‘deck chair sign’. Dermatologica 1986;172:65-6. [PubMed]
- Bech-Thomsen N, Thomsen K. Ofuji’s papuloerythroderma: a study of 17 cases. Clin Exp Dermatol 1998;23:79-83. [PubMed]
- Sugita K, Koga C, Yoshiki R, et al. Papuloerythroderma caused by aspirin. Arch Dermatol 2006;142:792-3. [PubMed]
- Sugita K, Kabashima K, Nakashima D, et al. Papuloerythroderma of Ofuji induced by furosemide. J Am Acad Dermatol 2008;58:S54-5. [PubMed]
- de Vries HJ, Koopmans AK, Starink TM, et al. Ofuji papuloerythroderma associated with Hodgkin’s lymphoma. Br J Dermatol 2002;147:186-7. [PubMed]
- Shimauchi T, Sugita K, Nakamura M, et al. Leukaemic cutaneous T-cell lymphoma-manifesting papuloerythroderma with CD3(-) CD4(+) phenotype. Acta Derm Venereol 2010;90:68-72. [PubMed]
- Guisado Vasco P, Fraile Rodríguez G, Barbolla Diaz I, et al. Comments on: Papuloerythroderma of Ofuji associated with chronic lymphatic leukaemia (Eur J Dermatol 2009; 19(4): 396-7). Eur J Dermatol 2010;20:418. [PubMed]
- Shimauchi T, Ohshima A, Tokura Y. Folliculotropic mycosis fungoides presenting as papuloerythroderma. J Dermatol 2006;33:498-500. [PubMed]
- Kim SW, Kang YS, Park SH, et al. A case of erythrodermic dermatomyositis associated with gastric cancer. Ann Dermatol 2009;21:435-9. [PubMed]
- Lonnee ER, Toonstra J, van der Putte SC, et al. Papuloerythroderma of Ofuji in a HIV-infected patient. Br J Dermatol 1996;135:500-1. [PubMed]
- Nomura T, Kodama K, Moriuchi R, et al. Papuloerythroderma of Ofuji associated with early gastric cancer. Int J Dermatol 2008;47:590-1. [PubMed]
- Chong VH, Lim CC. Erythroderma as the first manifestation of colon cancer. South Med J 2009;102:334-5. [PubMed]
- Kameyama H, Shirai Y, Date K, et al. Gallbladder carcinoma presenting as exfoliative dermatitis (erythroderma). Int J Gastrointest Cancer 2005;35:153-5. [PubMed]
- Sunami K, Taniguchi H, Moriyama T, et al. Papuloerythroderma associated with gastric cancer; report of a case. Nihon Shokakibyo Gakkai Zasshi 1995;92:1285-8. [PubMed]
- Watanabe M, Kimura T, Murasawa A. A case report of apatient with papuloerythroderma (Ofuji) and gastric cancer. Jpn J Clin Dermatol 2002;56:445-7.
- Nazzari G, Sabattini C. Ofuji’s papuloerythroderma. An association with early gastric cancer. Eur J Dermatol 1999;9:317-8. [PubMed]
- Schepers C, Malvehy J, Azón-Masoliver A, et al. Papuloerythroderma of Ofuji: a report of 2 cases including the first European case associated with visceral carcinoma. Dermatology 1996;193:131-5. [PubMed]
- Nishijima S. Papuloerythroderma associated with hepatocellular carcinoma. Br J Dermatol 1998;139:1115-6. [PubMed]


