Capecitabine maintenance therapy for XT chemotherapy-sensitive patients with metastatic triple-negative breast cancer
Introduction
In the last few years, ‘triple-negative’ breast cancer (TNBC) has been identified as a separate disease entity for its biological characteristics and clinical outcome. It is characterized by the presence of triple-negative immunohistochemistry (IHC) for estrogen receptor (ER), progesterone receptor (PgR) and human epidermal growth factor receptor 2 (HER2) (1). TNBC represents about 12-20% of all cases of breast cancer and occurs more frequently in young women; it is considered a subtype of basal-like disease, which has great variability of expression (2-5). Several studies have demonstrated that the prognosis is poor (6,7). TNBC has a highly aggressive nature, accounting for a disproportionate number of metastatic disease cases and breast cancer deaths (8-10). The majority of patients with TNBC have residual disease after treatment of early breast cancer. Further, they present a high risk of relapse and a sharp decrease in survival in the first 3-5 years after treatment (8,11-13). The peak risk of disease recurrence is within 3 years after treatment.
Metastatic TNBC (mTNBC) continues to represent a therapeutic challenge, as it is insensitive to some of the most effective therapies available for breast cancer treatment including HER2-directed therapy and endocrine therapy. The lack of known specific therapeutic targets results in limited resources to attack TNBC, which consist primarily of standard cytotoxic chemotherapy. In the metastatic setting, TNBC is associated with higher rates of visceral metastases, has a relatively shorter median survival of 7-13 months, and has limited duration of response to successive lines of chemotherapy (median response duration of 12 weeks to first line, 9 weeks to second, and 4 weeks to third line) (14-16). Therefore, it is important to select the agents most likely to result in a meaningful benefit and maintain a stable condition as long as possible.
Maintenance endocrine therapy is limited to metastatic breast cancer (MBC) patients with hormone receptor-positive disease (17,18). Maintenance therapy (MT) with targeted agents such as trastuzumab in HER2-positive MBC is a widely accepted treatment approach (19,20). However, the optimal duration of chemotherapy and the benefit of maintenance chemotherapy for mTNBC are still a matter of debate. In daily practice, maintenance chemotherapy is a reasonable strategy that prolongs time to progression in patients who do not show progression after initial chemotherapy. However this benefit should be considered together with treatment toxicities and the patient’s preference. To our knowledge, no MT for mTNBC is highly recommended yet. The present study focuses on the impact of capecitabine as maintenance for mTNBC.
Patients and methods
Patient selection
The major inclusion criteria included female patients aged ≥18 years with histologically confirmed ER–, PR–, and HER2– primary breast cancer (ER– and PR– were first defined as <10% positive tumor cells with nuclear staining in IHC and then <1% after April 2010 according to new College of American Pathologists guidelines at that time. HER2– was defined as IHC scoring 0 or 1+ or non amplified FISH according to the ASCO guidelines); patients must have at least one measurable lesion according to RECIST 1.1; an Eastern Cooperative Oncology Group (ECOG) score ≤2; no more than one-line prior treatment for advanced disease; patients who completed 4-8 cycles of XT combined chemotherapy and achieved disease control [complete relief (CR), partial relief (PR), or stable disease (SD)]. Patients were excluded if the original primary tumor or subsequent relapse was known to be positive for any of ER, PR or HER2.
Therapy methods
As both combination therapy and MT, capecitabine was administered at a dose of 900-1,000 mg/m2 twice daily on d 1-14, followed by a 7-d rest period. During MT, vitamin B6 (100 mg, 3 times daily) was co-administered to relieve the symptoms of hand-foot syndrome. In the combination regimens, docetaxel (XT) was administered at a dose of 75 mg/m2 as a 1-h intravenous infusion on d 1 of every 3-week cycle. After responding to chemotherapy, 32 patients continued to receive single-agent capecitabine at the same dose until disease progression or intolerable toxicity, and 23 patients were followed up without any treatment.
Efficacy and safety assessments
In total, the median progression-free survival (PFS) of 55 patients was studied, defined as the interval from the start of the treatment until disease progression, death or the last day of follow-up for patients who had not progressed at the date of analysis, for either XT or maintenance chemotherapies. Clinical efficacy and major adverse events were also studied. Response was assessed using the Response Evaluation Criteria in Solid Tumors guidelines (RECIST 1.1). Tumor size was evaluated by spiral computed tomography and magnetic resonance imaging. Adverse events were graded according to the National Cancer Institute Common Toxicity Criteria (NCI-CTC) version 4.0. Patients were followed up each cycle of chemotherapy for adverse events response assessment, and every two cycles for response evaluation.
Statistical analysis
PFS was estimated using the Kaplan-Meier method. Comparisons were performed using the log-rank test. Patient baseline characteristics and incidence of adverse events between capecitabine-MT group and non-MT group were compared using the Pearson χ2-test and t-test. Response rates of different groups were compared by chi-squared testing. All P values were from two-sided tests, and P<0.05 was considered statistically significant. Multivariate Cox survival regression analysis was conducted using a backward stepwise method (the statistical level of significance determined with the Wald test was P<0.05).The statistical data were obtained using an SPSS software package (SPSS 15.0 Inc., Chicago, IL, USA).
Results
Patient characteristics
A total 289 MBC patients received capecitabine plus XT chemotherapy between May 2007 and June 2013 at the Beijing Cancer Hospital. According to our inclusion criteria, 55 mTNBC patients with non-progressive disease (non-PD) to XT chemotherapy were eligible for analysis. After beneficial results from combined chemotherapy, 32 patients (58.2%) continued with capecitabine single-agent MT (MT group), and 23 patients (41.8%) were followed up without any treatment (non-MT group). All of the 55 patients were assessable for the efficacy and safety evaluation in this study, and no patient was lost to follow-up. The median age was 53 years (range, 34-71 years). The most common site of metastasis was lung (58.2%), and 70.9% of patients presented visceral metastasis. The majority of patients had received prior anthracycline-based chemotherapy (94.5%), with more than half (50.9%) receiving prior taxane-based chemotherapy. Thirty-five patients received XT as first-line chemotherapy for mTNBC, and second-line for 20 patients. In the MT group, patients received a median of 7.2 (range, 1.8-21.1) cycles of capecitabine maintenance treatment. Baseline characteristics of these patients are summarized in Table 1. No significant differences were detected between the two groups for baseline characteristics, such as median age, ECOG score, menstrual status, median disease-free survival (DFS), metastatic sites, number of metastatic lesions, and previous treatments. As of June 2013, the median follow-up time for 55 patients was 8.7 months (3.4-43.7 months), 10.3 months (4.1-35.9 months) in MT group, 8.1 months (3.4-43.7 months) in non-MT group, and there was no significant difference between two groups (P=0.622).
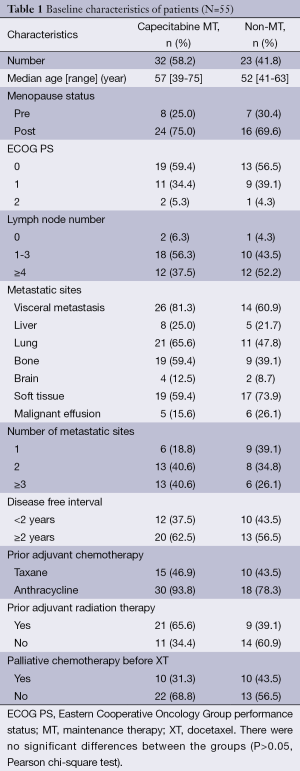
Full table
Therapeutic efficacy of XT chemotherapy-sensitive patients
Fifty-five patients received XT combined chemotherapy, with a median PFS of 8.1 months [95% confidence interval (95% CI), 6.9-9.3 months] with non-progressive response (Figure 1). Four (7.3%) patients received 8 cycles combined chemotherapy, 26 (47.3%) patients with 6 cycles, and 25 (45.5%) patients with 4 cycles of combined chemotherapy. The best response to combination chemotherapy was a PR in 22 patients (40.0%), and SD in 33 patients (60.0%). For 32 patients in the capecitabine MT group, 14 patients (43.8%) achieved a PR, and 18 patients (56.2%) had SD; while a PR in 8 patients (34.8%), and SD in 15 patients (65.2%) for 23 patients without MT (non-MT) group. The rates of PR and SD did not differ between two groups (P=0.503).
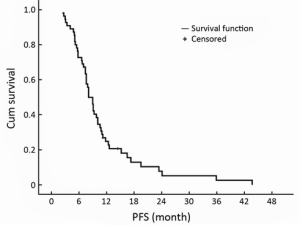
Median progression-free survival (PFS) of 55 mTNBC patients with capecitabine plus docetaxel (XT) combined chemotherapy. mTNBC, etastatic triple-negative breast cancer.
Therapeutic efficacy of capecitabine maintenance
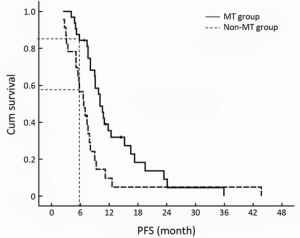
The median PFS time of XT chemotherapy plus capecitabine maintenance group was 10.1 months (95% CI, 8.7-11.5 months), and 6.7 months (95% CI, 4.8-8.6 months) for the XT chemotherapy without MT group (P=0.007, Figure 2). The 6-month PFS rates of two groups were significantly different (84.4% vs. 56.5%, P=0.032).
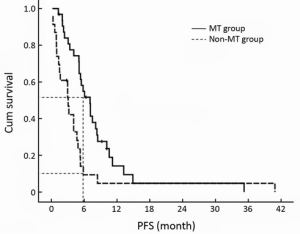
The PFS time of maintenance treatment defined by the duration of the beginning of single-agent capecitabine to disease progression, was 7.1 months (95% CI, 5.3-8.8 months) of stable condition after XT chemotherapy, but for non-MT group, the PFS time of non-treatment defined by the duration from the end of last cycle XT chemotherapy to disease progression was 3.1 months (95% CI, 1.3-4.8 months), and there was significant difference between both groups (P=0.003, Figure 3). Compared with non-MT, capecitabine maintenance treatment might prolong SD duration more than two fold. The 6-month PFS rate of maintenance treatment was 53.1%. For the non-MT group, only two patients maintained more than 6 months of no-progression time after chemotherapy cessation (53.1% vs. 8.7%, P=0.001).
Correlations between the PFS of the included patients with clinical features, such as number of lymph nodes, menstrual status, DFS more than 2 years or not, with or without visceral metastasis, number of metastatic lesions, number of chemotherapy cycles and clinical response rate were further analyzed by a multivariate Cox regression analysis, as shown in Table 2. The metastasis recurrence rate risk in the non-MT group was 2.517 times that of the MT group.
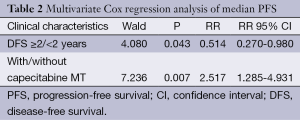
Full table
Toxicity analysis
A total of 275 cycles of XT chemotherapy were given with a median of 6 cycles (range, 4-10 cycles) in the MT group and 5 cycles (range, 4-8 cycles) in the non-MT group. The dose of capecitabine was modified in 6 patients for grade 3 hand-foot syndrome and interrupted in 2 patients because of grade 3 neutropenia. Table 3 lists the treatment-related toxicities (according to National Cancer Institute Common Terminology Criteria for Adverse Events) in the 55 patients assessed. Incidence of neutropenia, gastrointestinal toxicities, hand-foot syndrome and abnormal liver function were not significantly different between both groups. XT chemotherapy was well tolerated, and there were no grade 4 neutropenia or gastrointestinal toxicities in both groups. However, the incidence of hand-foot syndrome was higher in the capecitabine-MT group, but there was no significant difference (43.8% vs. 26.1%, P=0.179).
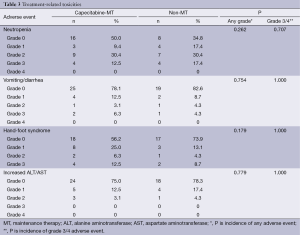
Full table
Discussion
Cytotoxic chemotherapy remains the mainstay of treatment for mTNBC. It is well known that once chemotherapy for metastatic disease is stopped, progression of the disease occurs quickly. For example, in the studies by Park et al. (21) and Alba et al. (18), progression occurred at a median time of 3.8 and 5.1 months after chemotherapy cessation, respectively. Therefore, MT for MBC patients was very important. Further more and more studies have focused on this topic recently. If a patient has a hormone receptor-positive tumor, most physicians would initiate treatment with maintenance hormonal therapy after the completion of chemotherapy, even though the precise role of this treatment has not been defined in a prospective randomized trial. But for those patients with TNBC, and rapidly proliferative and/or symptomatic disease, how to maintain the stable condition for a longer time is always a question. Recently a Korean Cancer Study Group (KCSG) phase III study reported that maintenance chemotherapy with paclitaxel (P) and gemcitabine (G) was associated with a statistically significant increase in the 6-month PFS, median PFS and overall survival for patients with HER2-negative MBC who had achieved disease control with six cycles of first-line PG chemotherapy (21), and patients with young age, TNBC, and visceral metastasis achieved more PFS benefits. Chemotherapy maintenance might be an effective treatment for TNBC patients.
The US NCI’s medical dictionary defines MT as a “type of treatment that is given to prevent progression after it has been controlled successfully with the initial therapy”. Aims of treatment in many advanced cancers are to prolong survival and improve patient’s quality of life. An effective MT should achieve both of these goals with a good patient tolerance, and lack of cumulative toxicities. Continuation maintenance where one component of initial therapy is continued after the induction treatment is always an option (22). In this context, capecitabine, one of the most widely used agents in MBC patients with a 20-28% response rate in taxane- and anthracycline-resistant patients and good tolerance (23,24), is proven one of the most suitable candidates for maintenance treatment.
Until now, clinical trials evaluating capecitabine-based regimens in TNBC are limited, and the results are inconsistent. In part, this may be because of the current interest in platinum compounds after anthracycline and taxane failure for this subtype. TNBCs are characterized by a deficiency in DNA repair machinery. Based on this background, it has been hypothesized that DNA alkyalting agents could be specifically effective in this subset of patients. A few retrospective studies or small sample neo-adjuvant trials have suggested that TNBC may be more sensitive to DNA-damaging agents such as CDDP (25,26), but others have shown contrary results (27). Nevertheless one phase II clinic trail suggested that cisplatin-based chemotherapy was superior to capecitabine-based regimen in the first-line treatment of mTNBC (28). However, it is too early to reach a conclusion on the efficacy of cisplatin in patients with TNBC, Furthermore, one of the shortcomings of this study was that capecitabine was stopped after six cycles of combination therapy and it is possible that capecitabine can play a role in maintenance treatment. XT and capecitabine are still among the most common choices in the first-line treatment of MBC. As an illustration of chemotherapy trials, O’Shaughnessy et al. (23) reported that adding capecitabine to XT provided a superior time to progression, overall survival and manageable side effects whatever the ER status. Against this rather negative background, we have reviewed our single-institution breast cancer database to assess the clinical efficacy of capecitabine-based chemotherapy against mTNBC. The results of the current study indicate that MT with single-agent capecitabine may prolong the duration of effective treatment for mTNBC patients who were sensitive to initial XT chemotherapy.
The duration of chemotherapy was debated for patients with MBC after a non-PD was achieved. In daily practice, 4-8 cycles of combined chemotherapy were frequently given before a cesarean section. In our study, 32 patients in MT group totally received 244 cycles of capecitabine maintenance chemotherapy (median 7.2 cycles). The overall PFS time and capecitabine maintenance PFS were 10.1 and 7.1 months in the MT group, respectively, both were significantly different from those of non-MT group (6.7 and 3.1 months, respectively). Apparently longer duration of chemotherapy prolonged PFS time. Several trials have reported that continuous chemotherapy prolongs the duration of remission, but its effect on survival and quality of life are less consistent (17,29,30). A recent meta-analysis of 11 randomized trials reported that a longer treatment duration of first-line chemotherapy was associated with a substantially longer PFS (21), and the relative benefits of tumor regression and improvement in disease-related symptoms provided by chemotherapy must be balanced with treatment-induced toxicity and its impact on quality of life. Therefore, it is crucial to choose proper agents for maintenance treatment. The MT group showed a higher incidence of hand-foot syndrome (43.8%), but mainly increased in grade 1 (25.0%). Longer treatment of capecitabine did not cause intolerable side effects. Capecitabine was an appropriate candidate for those patients who responded to initial XT chemotherapy. The results of the current study indicated that maintenance treatment with single-agent capecitabine was well tolerated. It is well known that oral drug administration avoids the need for central venous devices, and thus, reduces discomfort and risk of central venous catheter infection for patients, as well as hospitalization and administration costs, and improves patients’ quality of life.
Conclusions
In conclusion, our results indicate that single-agent capecitabine MT may prolong the PFS time for mTNBC patients who were sensitive to XT chemotherapy. Although no evidence of a specific sensitivity of TNBC has been reported, conventional chemotherapy was used in recent randomized trials on mTBNC patients. DNA alkylating agents, such as those containing platinum, were more interesting because of the BRCA1 mutation mechanisms in TNBC, however, none are capable of becoming MT. mTNBC patients are limited in terms of maintenance alternatives, but capecitabine may play a key role for MT and ensure good quality of life for patients.
Acknowledgements
The authors are grateful to Prof. Youyong Lu, from Beijing Institute for Cancer Research, Xing Zhou, from Peking University, and Xingjie Liang from the National Center for Nanoscience and Technology, who carefully reviewed for this manuscript.
Disclosure: The authors declare no conflict of interest.
References
- Perou CM. Molecular stratification of triple-negative breast cancers. Oncologist 2010;15 Suppl 5:39-48. [PubMed]
- Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature 2000;406:747-52. [PubMed]
- Sotiriou C, Neo SY, McShane LM, et al. Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc Natl Acad Sci U S A 2003;100:10393-8. [PubMed]
- Bidard FC, Conforti R, Boulet T, et al. Does triple-negative phenotype accurately identify basal-like tumour? An immunohistochemical analysis based on 143 ‘triple-negative’ breast cancers. Ann Oncol 2007;18:1285-6. [PubMed]
- Nielsen TO, Hsu FD, Jensen K, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res 2004;10:5367-74. [PubMed]
- Cheang MC, Voduc D, Bajdik C, et al. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res 2008;14:1368-76. [PubMed]
- Bauer KR, Brown M, Cress RD, et al. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer 2007;109:1721-8. [PubMed]
- André F, Zielinski CC. Optimal strategies for the treatment of metastatic triple-negative breast cancer with currently approved agents. Ann Oncol 2012;23 Suppl 6:vi46-51. [PubMed]
- Coughlin SS, Ekwueme DU. Breast cancer as a global health concern. Cancer Epidemiol 2009;33:315-8. [PubMed]
- Ismail-Khan R, Bui MM. A review of triple-negative breast cancer. Cancer Control 2010;17:173-6. [PubMed]
- Cheang MC, Voduc D, Bajdik C, et al. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res 2008;14:1368-76. [PubMed]
- Dent R, Hanna WM, Trudeau M, et al. Pattern of metastatic spread in triple-negative breast cancer. Breast Cancer Res Treat 2009;115:423-8. [PubMed]
- Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 2007;13:4429-34. [PubMed]
- Kassam F, Enright K, Dent R, et al. Survival outcomes for patients with metastatic triple-negative breast cancer: implications for clinical practice and trial design. Clin Breast Cancer 2009;9:29-33. [PubMed]
- Lin NU, Claus E, Sohl J, et al. Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer: high incidence of central nervous system metastases. Cancer 2008;113:2638-45. [PubMed]
- Sledge G, Miller K, Moisa C, et al. Safety and efficacy of capecitabine (C) plus bevacizumab (B) as first-line in metastatic breast cancer. J Clin Oncol 2007;25:abstr 1013.
- Gennari A, Amadori D, De Lena M, et al. Lack of benefit of maintenance paclitaxel in first-line chemotherapy in metastatic breast cancer. J Clin Oncol 2006;24:3912-8. [PubMed]
- Alba E, Ruiz-Borrego M, Margelí M, et al. Maintenance treatment with pegylated liposomal doxorubicin versus observation following induction chemotherapy for metastatic breast cancer: GEICAM 2001-01 study. Breast Cancer Res Treat 2010;122:169-76. [PubMed]
- Gennari A, Stockler M, Puntoni M, et al. Duration of chemotherapy for metastatic breast cancer: a systematic review and meta-analysis of randomized clinical trials. J Clin Oncol 2011;29:2144-9. [PubMed]
- Dufresne A, Pivot X, Tournigand C, et al. Maintenance hormonal treatment improves progression free survival after a first line chemotherapy in patients with metastatic breast cancer. Int J Med Sci 2008;5:100-5. [PubMed]
- Park YH, Jung KH, Im SA, et al. Phase III, multicenter, randomized trial of maintenance chemotherapy versus observation in patients with metastatic breast cancer after achieving disease control with six cycles of gemcitabine plus paclitaxel as first-line chemotherapy: KCSG-BR07-02. J Clin Oncol 2013;31:1732-9. [PubMed]
- Malik PS, Raina V, André N. Metronomics as maintenance treatment in oncology: time for chemo-switch. Front Oncol 2014;4:76. [PubMed]
- O’Shaughnessy J, Miles D, Vukelja S, et al. Superior survival with capecitabine plus docetaxel combination therapy in anthracycline-pretreated patients with advanced breast cancer: phase III trial results. J Clin Oncol 2002;20:2812-23. [PubMed]
- Blum JL, Dees EC, Vukelja SJ, et al. Phase II trial of capecitabine and weekly paclitaxel in patients with metastatic breast cancer previously treated with every-3-week taxane therapy. Clin Breast Cancer 2007;7:465-70. [PubMed]
- Sirohi B, Arnedos M, Popat S, et al. Platinum-based chemotherapy in triple-negative breast cancer. Ann Oncol 2008;19:1847-52. [PubMed]
- Silver DP, Richardson AL, Eklund AC, et al. Efficacy of neoadjuvant Cisplatin in triple-negative breast cancer. J Clin Oncol 2010;28:1145-53. [PubMed]
- Staudacher L, Cottu PH, Diéras V, et al. Platinum-based chemotherapy in metastatic triple-negative breast cancer: the Institut Curie experience. Ann Oncol 2011;22:848-56. [PubMed]
- Fan Y, Xu BH, Yuan P, et al. Docetaxel-cisplatin might be superior to docetaxel-capecitabine in the first-line treatment of metastatic triple-negative breast cancer. Ann Oncol 2013;24:1219-25. [PubMed]
- Falkson G, Gelman RS, Pandya KJ, et al. Eastern Cooperative Oncology Group randomized trials of observation versus maintenance therapy for patients with metastatic breast cancer in complete remission following induction treatment. J Clin Oncol 1998;16:1669-76. [PubMed]
- Nooij MA, de Haes JC, Beex LV, et al. Continuing chemotherapy or not after the induction treatment in advanced breast cancer patients. clinical outcomes and oncologists’ preferences. Eur J Cancer 2003;39:614-21. [PubMed]


