Expression of VEGFR2 and NRP-1 in non-small cell lung cancer and their clinical significance
Introduction
Mortality due to lung cancer ranks first among cancer types (1); thus it is critical to seek more effective lung cancer treatments. Currently, targeted anti-angiogenic therapy represents a new paradigm for patients with lung cancer; however, reports on its therapeutic effect vary. The reason for this variation may be due to differences (both clear and subtle) between individuals. As a consequence, research into how to select appropriate patients for corresponding treatments is warranted.
At present, the vascular endothelial growth factor- vascular endothelial growth factor receptor 2 (VEGF-VEGFR2) pathway is a major target of anti-angiogenic therapy. VEGFR2 is encoded by the kinase insert domain receptor (KDR) gene (located in 4q12) (2). As the predominant receptor for VEGF-stimulated endothelial cell functions, including cell migration, proliferation, survival, and enhancement of vascular permeability, VEGFR2 is mainly distributed in vascular endothelial cells. However, recent studies have shown that VEGFR2 is also present in malignant cells, including NSCLC (3) and breast cancer (4). In previous studies from our group, we observed varying degrees of VEGFR2 expression across several lung cancer cell lines, among which human lung squamous cell carcinoma (SCC) (Calu-1) cells had the highest expression.
Neuropilin-1 (NRP-1), similar to VEGFR2, is also a potential angiogenic target (5). NRP-1 expression has been reported on vascular endothelial cells in tumors as well as directly on the tumor cells in a study by Barr et al. (6). Some studies have confirmed that NRP-1 is a co-receptor that can enhance the binding of VEGF and VEGFR2, strengthening VEGF-induced endothelial cell chemotaxis, mitosis, and immunogenicity (5).
It is unclear whether VEGFR2 and NRP-1 might represent biomarkers that reflect the biological behavior and prognosis of NSCLC. We were also interested in exploring the relationship between VEGFR2 and NRP-1. In this study, we detected the expression of two biomarkers (VEGFR2 and NRP-1) in NSCLC tissues, analyzed their relationship, and investigated their roles in the development, metastasis and prognosis of NSCLC, which may provide guidance for clinical decision-making and anti-angiogenic therapy.
Material and methods
Materials
We randomly selected formalin-fixed and paraffin-embedded (FFPE) tissues from 40 NSCLC patients who underwent surgical resection in the department of thoracic surgery and pathologically and were diagnosed in our hospital (the First People’s Hospitial of Oncology, Lianyungang City, Jiangsu Province, China) between January 2009 and November 2012. During the same period, we also obtained ten cases of pulmonary inflammatory pseudotumor paraffin-embedded tissue specimens as controls. All paraffin block specimens were processed to 4-μm-thick serial sections.
Immunohistochemistry (IHC)
Separate slides were immunohistochemically stained for VEGFR2 and NRP-1. The primary antibodies (anti-VEGFR2 from Anbobio and anti-NRP-1 from Epitomics, USA) were prepared at a 1:200 dilution. The secondary antibody (PV kit) was purchased from Zhongshan Golden Bridge. To standardized judgement (7), the immunohistochemically stained tissues were evaluated by two experienced pathologists.
Fluorescence in situ hybridization (FISH)
FISH for VEGFR2 and NRP-1 was performed on the slides in separate experiments. Five microliters of FISH hybridization solution was added to each slide, and the probe system was prepared so that the proportion of the target gene vs. the control gene vs. the hybridization solution was 1:1:3 (Empire Genomics, USA). We then calculated the number of positive cells and tumor cells using a microscopic imaging and analysis system. Based on previously described judgment criteria (8), samples with an average of at least two gene copies per cell were considered copy number gain (CNG). Copy number analysis was performed in approximately 50 nuclei per tumor in at least five areas.
Statistical analysis
Data were input into SPSS16.0 statistical software to establish a database and were statistically processed. The Pearson chi-square test and Spearman rank correlation analysis were used where clinical information was considered. The distributions of overall survival (OS) and recurrence-free survival (RFS) were estimated using the Kaplan-Meier method and compared between groups using the log-rank test. Cox proportional hazard models were used for regression analyses of survival data and conducted on OS, defined as time from surgery to death or last contact, and on RFS, defined as time from surgery to recurrence or last contact. P<0.05 was considered statistically significant.
Results
VEGFR2 expression in NSCLC and benign lung lesions
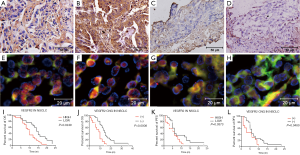
VEGFR2 was mainly located at the cell membrane and in the cytoplasm of tumor cells and tumor stromal vascular endothelial cells. VEGFR2 was not expressed in samples from ten benign lung lesions. However, samples from 23 of 40 NSCLC patients exhibited high expression, and 17 had low expression (Figure 1A-D). The rate of high expression was 58% (23/40), which is statistically significant compared with the rate in benign lung lesions (χ2=10.65, P=0.001). KDR CNG was not observed in samples from 10 benign lung lesions, but of the 40 NSCLC patient samples, there were 13 cases with CNG and 27 with no CNG (Figure 1E-H). The rate of CNG was 32.5% (13/40), which was statistically significant compared with the rate in benign lung lesions (χ2=4.39, P=0.036).
NRP-1 expression in NSCLC and benign lung lesions
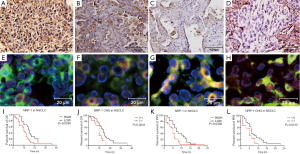
NRP-1 was expressed mainly in the cytoplasm of tumor cells and endothelial cells and was marginally present near the membrane. NRP-1 was not expressed in the 10 benign lung lesion samples, but 22 of 40 NSCLC patient samples had high expression and 18 had low expression (Figure 2A-D). The rate of high expression was 55% (22/40), which was statistically significant compared with the rate in benign lung lesions (χ2=9.82, P=0.002). No NRP-1 CNG was observed in the samples from 10 benign lung lesions, but of the 40 NSCLC patient samples, 12 cases had CNG and 28 did not (Figure 2E-H). The rate of CNG was 30% (12/40) in NSCLC, which was statistically significant compared with the rate in benign lung lesions (χ2=3.95, P=0.047).
Relationship between VEGFR2 and NRP-1 protein expression and CNG and clinicopathological parameters
The protein expression and gene levels of VEGFR2 and NRP-1 were associated with tumor TNM stage, lymph node metastasis and tumor differentiation (P<0.05) but not with gender, age or pathological type (P>0.05) (Tables 1,2).
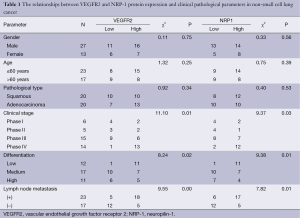
Full table

Full table
Correlation between VEGFR2 and NRP-1 expression in lung cancer
In NSCLC, the protein expression of VEGFR2 and NRP-1 showed a positive correlation (Rs=0.50, P=0.00); a correlation was also observed between the CNG of the two genes (Rs=0.20, P=0.00). Survival analysis demonstrated that the median OS of patients classified as having high and low VEGFR2 expression was 10 and 14 months, respectively; the corresponding rates were 10 and 14.5 months for patients with high and low NRP-1 expression, respectively. The median survival was 9 and 14 months for KDR CNG (+) and CNG (−) patients, respectively, and 10 and 14 months for NRP-1 CNG (+) and CNG (−) patients, respectively (P<0.05) (Figure 1, Figure 2I-L and Table 3).
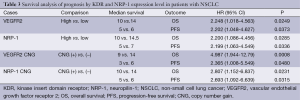
Full table
Discussion
This study explored VEGFR2 protein expression in 40 cases of NSCLC. The rate of high expression was 58%, whereas no expression was detected in benign pulmonary lesions. A statistically significant difference was observed between the two groups (χ2=10.65, P=0.001). VEGFR2 is the specific receptor for VEGF. Binding of VEGF to VEGFR2 leads to endothelial cell division and proliferation through a series of regulatory mechanisms and biofeedback. Binding also promotes endothelial cell migration and increased capillary permeability and plasma exudation. VEGFR2 overexpression accelerates the tumor growth process. However, it is unclear whether the KDR gene is also amplified in patients with high protein expression. Therefore, we performed FISH detection. Among the 40 cases of NSCLC, KDR CNGs were detected at a rate of 32.5%, while no CNG was observed in the benign lung lesions, revealing a statistically significant difference (χ2=4.39, P=0.036). The study demonstrated that VEGFR2 protein levels were closely associated with the tumor TNM stage, lymph node metastasis and tumor differentiation (P<0.05) but had no apparent relationship with age or pathological type (P>0.05), consistent with the report of Jiang (7). Significant KDR CNG (+) was observed in the NSCLC group compared with the control group (χ2=4.39, P=0.036), consistent with the report of Yang et al. (9). Our results indicated that high expression of KDR had no clear relationship with gender, age, or pathological type (P<0.05) but was closely related to lymph node metastasis, clinical stage and the degree of tumor differentiation (P>0.05). However, previous studies have shown that KDR expression was associated with tumor histological type. Brown et al. (10) reported that KDR was highly expressed in acne type ductal carcinoma in situ, invasive ductal carcinoma in situ, ductal carcinoma and metastatic endothelial cells in breast cancer but was not present in invasive lobular carcinoma. His results are not completely consistent with ours, possibly due to the difference in histological source and specimen constitution.
At present, IHC is a commonly used method but can easily lead to false negative or false positive results because it is highly affected by evaluator subjectivity. FISH represents a recent development in molecular biology techniques. FISH analysis generates accurate results that can be quantified, but the technique has the disadvantage of being relatively expensive. In this study, we determined the KDR gene status in 40 cases of NSCLC patients by FISH and executed comparative analyses in combination with the IHC results. We observed that 6 cases were positive by FISH among the 9 determined to be strongly positive (+++) by IHC; the positive coincidence rate reached 67%. We detected seven cases as positive by FISH among the 14 patients determined to be positive (++) by IHC; the positive coincidence rate was only 50%. When specimens were judged as (+) or (−) by IHC, the FISH results also indicated no amplification. KDR CNG can largely be ruled out. Therefore, we believe that the results in cases previously screened as (+++) by IHC appear consistent with the FISH analysis. However, for patients judged as (++), the coincidence rate of two methods was too low. Before targeted drug therapy is implemented, the KDR gene status should be defined by FISH.
NRP-1 is known to be involved in angiogenesis and has furthermore been identified as a novel type of receptor. Research on the anti-angiogenic effects of VEGF and NRP-1 has gradually increased. Previous studies have reported that NRP-1 is abnormally increased in gastric cancer, breast cancer, esophageal cancer, prostate cancer and other tumor tissue and cancer cells, and NRP expression is closely related to the occurrence of tumors (11-14). Our study also explored NRP-1 protein expression in 40 NSCLC cases. The rate of high NRP-1 expression was 55%, and no expression was detected in benign pulmonary lesions; the difference between the two groups was statistically significant (χ2=9.82, P=0.002). Among the 40 NSCLC samples, NRP-1 CNG (+) was detected in 32% of cases, while no CNG was detected in benign lung lesions, revealing a statistically significant difference (χ2=3.95, P=0.047). The study demonstrated that NRP-1 protein levels were closely associated with the tumor TNM stage, lymph node metastasis and degree of differentiation (P<0.05) but had no apparent relationship with age or pathological type (P>0.05). Our results do not completely correspond to the observations of Liu (15). In that study, NRP-1 expression was not associated with degree of tissue differentiation. This discrepancy may be due to the smaller sample size in this study. In addition, reports of NRP-1 CNG in NSCLC are rare. We simultaneously detected NRP-1 gene status in 40 cases of NSCLC by FISH and performed a comparative analysis with IHC results. We found that five cases were positive by FISH among the seven patients determined to be strongly positive (+++) by IHC; the positive coincidence rate was 71%. We detected seven cases as positive by FISH among the 15 patients determined to be positive (++) by IHC; the positive coincidence rate was only 47%. When specimens were judged as (+) or (−) by IHC, the FISH results indicated no amplification, and NRP-1 CNG could largely be ruled out. Therefore, we also believe that the results in cases first screened as (+++) by IHC appeared consistent with the results of FISH. However, for patients with (++) expression levels by IHC, the two methods illustrated a low coincidence rate. Before targeted drug therapy is implemented, NRP-1 gene status should be defined by FISH.
In addition, to further study the relationship between VEGFR2 and NRP-1, we also conducted a Spearman rank correlation analysis. We found that VEGFR2 and NRP-1 were both highly expressed in NSCLC tissues at the protein level, and expression of the two proteins was positively correlated (Rs=0.50, P=0.00). These results are in line with the previous report of Zhou (16), which noted that when NRP-1 and VEGFR2 are co-expressed in endothelial cells, NRP-l enhanced the VEGF and VEGFR2 interaction, resulting in increased VEGF-mediated endothelial cell chemotaxis and the promotion of mitosis. Conversely, inhibiting the interaction between VEGF and NRP-1 would suppress VEGF and VEGFR2 binding, restraining VEGF-induced mitotic activity. At the gene level, KDR and NRP-1 CNGs were detected in NSCLC tissues and were positively correlated (Rs=0.20, P=0.00). This finding has not been reported previously. We believe that VEGFR2 expression is positively correlated with NRP-1, implying that NRP-1 may play a key role in promoting the interaction between VEGF and VEGFR2. NRP-1 induces the formation of new blood vessels in tumor tissue and is involved in the growth, infiltration and metastasis of NSCLC tissue. However, many studies on VEGFR2 and NRP-1 expression have been conducted, but the conclusions have not been consistent. Research on the joint detection of VEGFR2 and NRP-1 and analysis of the correlation of their expression in NSCLC has not been conducted, and the specific relationship between VEGFR2 and NRP-1 still needs to be further explored.
Estimated survival rates are important for judging the prognosis of patients with NSCLC. The ability to predict survival in lung cancer may benefit treatment and improve prognosis. This study found that OS (P=0.0249) and progression-free survival (PFS) (P=0.0373) in the group with low VEGFR2 expression were higher than in the group with high expression. This finding differs from that reported by Holzer et al. (17), in which OS was not significantly different between these two groups. This may be due to the retrospective nature of the present analysis, the relatively small sample size or differences in clinical treatments. The results warrant further prospective studies with larger sample sizes to confirm the findings. However, OS (P=0.0008) and PFS (P=0.0480) were significantly different between the VEGFR2 CNG (+) and CNG (−) groups, in line with a report by Yang et al. (9). We also found that OS (P=0.0285) and PFS (P=0.0336) were higher in the low NRP-1 expression group compared with the high expression group. In addition, OS (P=0.0231) and PFS (P=0.0315) were significantly different between the NRP-1 CNG (+) and CNG (−) groups. Research analyzing the association between survival and NRP-1 levels in patients with NSCLC has not been reported previously. However, in head and neck tumors, as described by Xu et al. (18), among patients with nasopharyngeal carcinoma (NPC), high NRP-1 expression was closely associated with advanced clinical stage (P=0.02), positive recurrence (P=0.001) and metastasis status (P=0.001), and patients with high NRP-1 expression suffered from shorter OS (P=0.001). Mehta et al. (19) reported that in patients with SCC of the head and neck, negative NRP-1 status was associated with longer OS (P=0.04). Thus, additional long-term research may ultimately verify our experimental results.
In conclusion, while the precise effects and mechanism of action of VEGFR2 and NRP-1 in NSCLC during tumor initiation, infiltration and metastasis are gradually being verified, VEGFR2 and NRP-1 can provide screening biomarkers for anti-angiogenic therapy in lung cancer. Both exhibited some associations with the survival and prognosis of patients with NSCLC. Anti-VEGFR2 and anti-NRP-1 therapy may have potential in cancer treatment and can serve as channels to provide a theoretical basis for identifying new therapeutic avenues for the clinic that will be beneficial to the majority of patients.
Acknowledgements
Funding: This work was supported by National Natural Science Foundation of China [81472792], Ministry of Health of China (W201210), Jiangsu Natural Science Foundation of China (BK2012661).
Disclosure: The authors declare no conflict of interest.
References
- Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277-300. [PubMed]
- Terman BI, Carrion ME, Kovacs E, et al. Identification of a new endothelial cell growth factor receptor tyrosine kinase. Oncogene 1991;6:1677-83. [PubMed]
- Donnem T, Al-Shibli K, Andersen S, et al. Combination of low vascular endothelial growth factor A (VEGF-A)/VEGF receptor 2 expression and high lymphocyte infiltration is a strong and independent favorable prognostic factor in patients with nonsmall cell lung cancer. Cancer 2010;116:4318-25. [PubMed]
- Seto T, Higashiyama M, Funai H, et al. Prognostic value of expression of vascular endothelial growth factor and its flt-1 and KDR receptors in stage I non-small-cell lung cancer. Lung Cancer 2006;53:91-6. [PubMed]
- Soker S, Fidder H, Neufeld G, et al. Characterization of novel vascular endothelial growth factor (VEGF) receptors on tumor cells that bind VEGF165 via its exon 7-encoded domain. J Biol Chem 1996;271:5761-7. [PubMed]
- Barr MP, Byrne AM, Duffy AM, et al. A peptide corresponding to the neuropilin-1-binding site on VEGF(165) induces apoptosis of neuropilin-1-expressing breast tumour cells. Br J Cancer 2005;92:328-33. [PubMed]
- Jiang XD, Dai P, Song DA, et al. Expressions of HIF-1α, VEGF and VEGFR2 in Non-small Cell Lung Cancer and their Clinical Significance. Journal of Clinical Pulmonary Medicine 2011;16:386-8.
- Tang X, Kadara H, Behrens C, et al. Abnormalities of the TITF-1 lineage-specific oncogene in NSCLC: implications in lung cancer pathogenesis and prognosis. Clin Cancer Res 2011;17:2434-43. [PubMed]
- Yang F, Tang X, Riquelme E, et al. Increased VEGFR-2 gene copy is associated with chemoresistance and shorter survival in patients with non-small-cell lung carcinoma who receive adjuvant chemotherapy. Cancer Res 2011;71:5512-21. [PubMed]
- Brown LF, Berse B, Jackman RW, et al. Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in breast cancer. Hum Pathol 1995;26:86-91. [PubMed]
- Starzec A, Vassy R, Martin A, et al. Antiangiogenic and antitumor activities of peptide inhibiting the vascular endothelial growth factor binding to neuropilin-1. Life Sci 2006;79:2370-81. [PubMed]
- Gong Y, Hu GH, Tang WX, et al. The effect of NRP-1 on proliferation, invasion and tumor angiogenesis of human laryngocarcinoma cell line Hop-2. Journal of Chongqing Medical University 2008;33:1479-83.
- Zhang J, Zhang SQ, Dong P, et al. The expression changes and significance of VEGF and N R P-1 in laryngeal squamous carcinoma tissues. Shandong Medical Journal 2010;50:95-6.
- Alattar M, Omo A, Elsharawy M, et al. Neuropilin-1 expression in squamous cell carcinoma of the oesophagus. Eur J Cardiothorac Surg 2014;45:514-20. [PubMed]
- Liu HX. Expression of Neueropilin-1 in Non-small Cell Lung Cancer and Its Clinical Significance. Practical Preventive Medicine 2011;18:1941-3.
- Zhou D, Wang HB. The Relationship Between Neuropilin-1 and Tumor Angiogenesis. Journal of International Obstetrics and Gynecology 2009;36:263-6.
- Holzer TR, Fulford AD, Nedderman DM, et al. Tumor cell expression of vascular endothelial growth factor receptor 2 is an adverse prognostic factor in patients with squamous cell carcinoma of the lung. PLoS One 2013;8:e80292. [PubMed]
- Xu Y, Li P, Zhang X, et al. Prognostic implication of neuropilin-1 upregulation in human nasopharyngeal carcinoma. Diagn Pathol 2013;8:155. [PubMed]
- Mehta S, Moon J, Hashmi M, et al. Predictive factors in patients with advanced and metastatic squamous cell carcinoma of the head and neck: a study based on SWOG protocol S0420. Oncol Rep 2013;29:2095-100. [PubMed]


