Factors contributing to lymph node occult metastasis in supraglottic laryngeal carcinoma cT2-T4 N0M0 and metastasis predictive equation
Introduction
Laryngeal carcinoma (LC) accounts for 13.9% of head and neck cancers, with an incidence of 1.5-3.4 per 100,000; its incidence tends to increase year on year. The prognosis is generally good for LC, with 5-year survival rates of 70-80% for T2 lesions and 40-60% for T3-T4 lesions in the absence of distant metastasis (1). Therefore, the minimization of surgical treatment and the restoration of function are major trends in the treatment of LC, on the supposition that the relapse risk will not be increased.
The importance of neck dissection has been established as a surgical intervention technique in the treatment of supraglottic laryngeal carcinoma (SLC), especially for advanced cases; it has evolved from radical neck dissection and modified neck dissection to the more acceptable selective neck dissection, which is presently used. Currently, most patients who do not have clinical lymph node metastasis (LNM) from SLC choose selective neck dissection as the first-line surgical intervention. However, no LNM has been found in many patients with cT2-T4 N0M0 (cN0) SLC following selective neck dissection, or in many patients who have not received selective neck dissection during long-term follow-up. Despite its limited impairment effects, selective neck dissection results in postoperative injuries of diverse intensity in almost all patients. These include: hematomas, accessory nerve damage, lymphatic fistulas, compromised cosmetic appearance, scar discomfort, facial swelling, and local sensorimotor dysfunction. These injuries all diminish the patient’s quality of life (2). Although the injuries induced by radical neck dissection can be minimized using selective neck dissection, local structures must be preserved. This requires more careful intraoperative procedures, increased anesthesia time and a precise surgical technique. Because of the size and position of the lesions treated with neck dissection, the probability of neck infection is expected to increase. Furthermore, cervical dissection can produce scars that might adversely affect the efficacy of radiotherapy, thus decreasing the effective radiation dose delivered to the target region. All of these factors affect the prognosis of patients with SLC. It remains unclear whether neck dissection should be carried out for advanced SLC.
In the present study, 121 patients with grade cN0 SLC admitted to Beijing Tongren Hospital from December 2002 to January 2013 were analyzed to investigate the importance of cervical dissection for different stages of cN0 SLC.
Materials and methods
General data
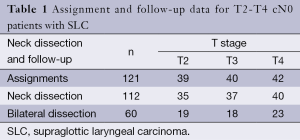
A total of 121 patients with cN0 SLC who underwent surgical intervention in Beijing Tongren Hospital from December 2002 to January 2013 were enrolled in this study. There were 110 men and 11 women with a median age of 61 (range, 41-80) years. Their identified tumor stages were T2 (n=39), T3 (n=40) and T4 (n=42). All patients met the diagnosis criteria and underwent no preoperative radiotherapy or chemotherapy (Table 1).
Full table
Diagnostic criteria
LC was diagnosed based on the 2002 TNM Staging Classification System (UICC). The cN0 SLCs were diagnosed using the evaluation criteria proposed by Kowalski et al. (3): (I) clinical examination revealed that lymph nodes were <2 cm in diameter and were soft; and (II) radiography found that no lymph nodes were >1 cm in diameter. All the patients received an enhanced neck CT scan or a neck ultrasound examination, and the radiography results showed that there were no lymph nodes >1 cm in diameter.
Treatment
Fifty-five patients underwent total laryngectomy and 66 patients underwent partial laryngectomy. The patients who did not undergo neck lymph node dissection met the requirements of CT findings, which suggested that their neck lymph nodes were <1 cm in diameter and of uniform density. The 112 patients (172 sides) received cervical lymph node dissection at levels II, III or IV simultaneously during the laryngectomies, including 37 patients with stage T2, 38 with stage T3 and 37 with stage T4 disease. If the cancer lesion was located mainly on one side of the larynx and analysis of the frozen sections revealed there was no lymphatic metastasis on the major side, we carried out unilateral neck dissection; if it was not located mainly on one side of the larynx or the frozen sections revealed there was lymphatic metastasis on the major side, bilateral neck dissection was performed. Nine patients did not undergo any cervical lymph node dissection because of poor physical condition or personal wishes (Table 1). According to the condition of their lesions, pathological findings and personal conditions, 58 patients received postoperative radiotherapies involving total radiation doses of 5,000-7,000 cGy.
Patient follow-up
Patients were followed up from the date of intervention to September 2013 every sixth months. The median follow-up time is 49 months and the median survival time was 9 years. Six patients underwent surgery within 1 year before September 2013 and were available for follow-up. A total of 115 patients were followed up for >1 year, including 48.7% (56/115) of the patients who had received postoperative radiotherapies.
Statistical analysis
The SPSS 17.0 software package (SPSS Inc., Chicago, IL, USA) was used for statistical analysis of the follow-up data. The primary endpoint was death or the follow-up cutoff date. The postoperative survival rate was estimated using the life table method. The log-rank method (Backwards: LR) was used to compare intergroup differences in survival rates between patients with LNM and those without LNM. A logistic regression model (Backwards: LR) was used for multivariate analysis of LNM; α=0.05 was considered the significance level in the hypothesis test.
Results
LNM rate
Of the 121 patients enrolled in the study, 34 (28.1%) had metastasis, and there were 68 positive nodes out of a total of 3,359 lymph nodes revealed during neck dissection.
Regional distribution of LNM
Of the 112 patients who underwent lymphadenectomy, 22 (19.6%) had level II metastasis, 21 (18.8%) had level III metastasis and 4 (3.6%) had level IV metastasis. The patients with level IV LNM also had level III ipsilateral LNM, and 2 patients with level IIB LNM had level IIA ipsilateral LNM. Eight patients (1 with T2, 2 with T3 and 5 with T4 disease) had metastasis at more than one level.
Correlation between LNM rate and its affected factors
Correlation between LNM rate and T-staging in LC
Of the 34 patients with metastasis, 6 had T2 disease and the incidence of occult metastatic T2 disease was 15.4% (6/39); 13 patients had T3 disease and the incidence of occult metastasis from T3 disease was 32.5% (13/40), including 2 patients with bilateral cervical LNMs. Fifteen patients had T4 disease and the incidence of occult metastasis from T4 disease was 35.7% (15/42), including two patients with bilateral cervical LNMs. Chi-square test between LNM rate and T-staging showed that P=0.095. Sixteen patients (2 with T2, 4 with T3 and 10 with T4 disease) had more than one metastatic lymph node.
Correlation between LNM rate and classification of laryngeal pathology
Metastasis rates were 7.7% (2/26), 34.7% (26/75) and 30.0% (6/20) for lesions with high, moderate and poor differentiation, respectively. Chi-square test between LNM rate and pathological classification showed that P=0.030.
Multivariate analysis of LNM data
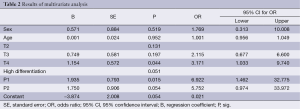
A logistic regression model (Backwards: LR) was used for multivariate analysis of LNM data, and age, sex, T-staging and pathological classification were included as covariates. The logistic regression model was statistically significant (P=0.032). T-staging and pathological classification, but not age and sex, were correlated with LNM (Table 2). The odds ratios (ORs) for T3/T2, T4/T2 and T4/T3 stage disease were 2.115, 3.171 and 1.499, respectively. Comparisons of the pathology results were as follows: moderate vs. high differentiation, OR =6.922; poor vs. high differentiation, OR =5.752; and poor vs. moderate differentiation, OR =0.831. The regression equation used for predicting the probability (Pn) of metastasis in cN0 SLC was:
Full table
Pn = e(–3.874+0.749T3+1.154T4+1.935P1+1.750P2)/[1+e(–3.874+0.749T3+1.154T4+1.935P1+1.750P2)]
T3, T4, P1 (moderate differentiation) and P2 (poor differentiation) had values of 0 or 1 according to the patient; the above four parameters could be obtained before surgery. The receiver-operating characteristic (ROC) curve (Figure 1) for the predicted probability of metastasis showed that the area under the curve (AUC) was 0.712 (95% CI: 0.614-0.810), and the diagnostic performance of the regression equation was good. The Pn was 0.3422 (Se =0.765, Sp =0.598) when the Youden index was at its highest, and indicated that when Pn >0.3422, a risk of metastasis existed.

Incidence of postoperative LNM and survival rates
Of the 115 patients who were followed up after surgery for >1 year, 2 who were confirmed as having no LNM had cervical LNM with no concomitant recurrence of SLC, 1 who had T3 disease and subglottic invasion had ipsilateral IV and VI lymph nodes and neck soft tissue metastases, and another patient who had T4 disease had ipsilateral VI metastasis. All nine patients who did not undergo neck dissection were followed up for >4 years, and two had recurrence of SLC, but none LNM.
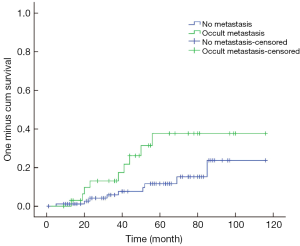
The 3- and 5-year survival rates were 92% and 81%, respectively, for all patients; this compared with 84% and 61%, respectively, for patients with LNM. The intergroup comparison of survival curves (Figure 2) between patients with and without LNM was statistically significant (P=0.029).
At the end of the follow-up period, 26 deaths were reported, 19 of which resulted from SLC-related factors. Among these 26 patients, 10 deaths were related to lung metastasis, 5 to topical recurrence and 4 to pulmonary infection. Other causes of death included metastasis to other sites such as the liver, brain and kidney, depression, eating difficulties and systemic failure.
Discussion
Complete resection of primary lesions and cervical dissection are the main interventions involved in the comprehensive treatment of SLC, and critically affect its prognosis. Although the advantage of dissection for patients with cervical LNM is well established, the necessity of cervical dissection in patients with cN0 SLC remains controversial (4,5).
Neck dissection should be necessary if the risk of cervical LNM is >15-20% (6). Supraglottic and glottic LCs are common specimens of this disease, as shown by clinical data. Supraglottic structures are enriched with lymph ducts that are connected to each other. The rate of metastasis to the cervical lymph nodes from SLC has been reported to be 10-50%, with an average rate of ~33%. Therefore, selective neck dissection is generally recommended for SLC (7,8). However, some studies have shown no significant survival benefit for patients with cN0 SLC following cervical dissection and radiotherapy, compared with patients who underwent observation only (5). In the present study, 112 patients with cN0 SLC received selective cervical dissection. As shown in postoperative pathology examinations, 28.1% were confirmed as having metastasis. Metastasis rates for T2, T3 and T4 lesions were 15.4%, 32.5% and 35.7%, respectively. The metastasis rate for cervical lymph nodes with cN0 SLC appears to be closely related to T-staging in patients with SLC, and tends to increase with the progress of T-staging. As shown by multivariate analysis, T-stage and pathology classification were correlated to LNM. The LNM rate for T3 was 211.5% higher than for T2, the T4 rate was 317.1% higher than for T2, and the T4 rate was 149.9% higher than for T3. Consequently, neck dissection is necessary for advanced SLC according to these data. The LNM rate increased by a factor of 6.922 in moderately differentiated cases compared with highly differentiated cases, by a factor of 5.752 in poorly differentiated cases compared with highly differentiated cases, and by a factor of 0.831 in poorly differentiated cases compared with moderately differentiated cases. These observations might reflect the fact that the worse the differentiation of the lesion, the greater the opportunity for the development of LNM. The finding that the LNM rate increased by a factor of 0.831 in poorly differentiated cases compared with moderately differentiated cases may be related to the fact that poorly differentiated lesions would metastasize in their early stages; most of the poorly differentiated cases presented as distinct (N+) or even severe LNM. In the moderately differentiated and poorly differentiated cases, we should pay more attention to the lymph nodes during follow-up. According to the multivariate analysis, we formulated an equation that may be used to predict the possibility of LNM in cN0 SLC patients. Although an equation for the prediction of the possibility of LNM has not been previously presented and its rationale needs to be tested, the equation that we formulated predicted 70% of cases with LNM in cN0 SLC before surgery. Therefore, prophylactic cervical dissection is necessary for the treatment of advanced cN0 SLC, especially when Pn is >0.3422.
Currently, cervical lymph node dissection usually focuses on levels II, III and IV (8-10). However, recent studies have also shown instances of rare metastasis to level IIb and IV (11,12). Other studies have demonstrated that prophylactic bilateral cervical dissection is not always required for patients with cN0 SLC with high metastatic potential, and the dissection range should be determined according to the lesion and the results from the evaluation of intraoperative frozen sections (13). Statistical analyses of regional metastasis in the current study identified levels II and III as the main regions of metastasis; metastasis rates from SLC were 19.6%, 18.8% and 3.6% for levels II, III and IV, respectively. There were no obvious rules to follow regarding metastasis between levels II and III. However, level IIB LNMs in our study were concomitant with ipsilateral IIA LNMs. Patients with no IIA metastasis also had no IIB metastasis. Level IV LNMs were all concomitant with ipsilateral level III LNMs. Patients with no ipsilateral level III LNM also had no level IV LNM. Four contralateral LNMs were advanced T3 and T4 lesions, which all passed over center lines; ipsilateral metastasis predominated in LNMs. These results indicate that cervical LNM might have some characteristics of sequential metastasis, which reflect the extension of local lymphatic drainage. SLC could metastasize simultaneously to level II and III. Metastasis to level II might originate from level IIA, without jumping to level IIB directly; similarly, metastasis to level IV might originate from level III, without jumping to level IV directly. In the absence of ipsilateral level II or III metastasis, contralateral metastasis is infrequent. In conclusion, the range of prophylactic neck dissection used for the treatment of cN0 SLC should be based on examination of intraoperative frozen sections. For example, dissection of level IIB metastasis might be unnecessary in the absence of level IIA metastasis. In addition, dissection of level IV metastasis might be unnecessary in the absence of level III metastasis, and contralateral dissection might be unnecessary in the absence of ipsilateral metastasis.
Clinical data have demonstrated that the recurrence of metastasis in the cervical lymph nodes is common in the first 3 postoperative years, with the highest incidence in the first year (14). The survival rate of patients with LNM was significantly lower than those without such metastasis (15). In our study, 115 patients were followed for >1 year. Two patients with no postoperatively confirmed LNM experienced cervical LNM without SLC recurrence; both lesions were identified as advanced lesions, mainly with levels VI and IV as the dominant metastasis sites. Therefore, for patients with advanced disease, especially those with subglottic involvement, assessment and dissection at levels VI and IV should be seriously considered.
Moreover, improvement of preoperative diagnostic accuracy for cervical LNM from LC has become a research highlight. Currently, commonly used approaches such as CT, B-ultrasound and cervical lymph node biopsy have some limitations. Recently, certain biomarkers of tumor tissues, such as ILV, VEGF-C, VEGF-C/VEGFR-3, osteopontin, PTEN, thrombospondin2, HIF-1α, CXCR2, E-cadherin FAK and MMP, have been correlated with LNM, and are therefore worth investigating (16-19). In addition, the regression equation for the predicted probability of metastasis from SLC cN0 formulated in our study provides a new noninvasive method for predicting LNM before surgery. These findings require further investigation.
Conclusions
Based on our data analysis, unilateral lymph dissection might be considered as a treatment for the involved T2 region of cN0 SLC. Results from the examination of cryosections of the lesion-involved lymph nodes can indicate whether contralateral lymph node dissection should be considered for the treatment of T3 and T4 cN0 SLC. Level IIB dissection is not advisable for patients with no level IIA lymph metastasis. Lymph node dissection in level IV might not be immediately advisable for patients with T2 and T3 SLC with no intraoperative level III metastasis. For patients with advanced disease, especially in the case of lesions that involve the subglottic region, assessment or exploration of level VI lymph nodes should be considered. Therefore, it is preferable to undertake prophylactic cervical dissection for the treatment of advanced cNO SLC when the Pn is >0.3422 using our predictive equation.
Acknowledgements
Funding: This work was supported by “Beijing City, the hospital authority clinical technology innovation project (Grant No. XMLX201311)”, “The Beijing Municipal Science and Technology Commission capital characteristic clinical application research project (Grant No. Z141107002514003)” and “Special Research Found for the Doctoral Program of Higher Education (Grant No. 20121107110021)”.
Disclosure: The authors declare no conflict of interest.
References
- Jia SS, Xiang C, Liu WS, et al. Supraglottic horizontal partial laryngectomies in 163 cases. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2006;41:763-6. [PubMed]
- Osuch-Wójcikiewicz E, Chęciński P, Bruzgielewicz A, et al. The advanced hypopharyngeal cancers surgery treatment complications. Otolaryngol Pol 2011;65:73-7. [PubMed]
- Kowalski LP, Bagietto R, Lara JR, et al. Prognostic significance of the distribution of neck node metastasis from oral carcinoma. Head Neck 2000;22:207-14. [PubMed]
- Canis M, Plüquett S, Ihler F, et al. Impact of elective neck dissection vs observation on regional recurrence and survival in cN0-staged patients with squamous cell carcinomas of the upper aerodigestive tract. Arch Otolaryngol Head Neck Surg 2012;138:650-5. [PubMed]
- Goudakos JK, Markou K, Nikolaou A, et al. Management of the clinically negative neck (N0) of supraglottic laryngeal carcinoma: a systematic review. Eur J Surg Oncol 2009;35:223-9. [PubMed]
- Pillsbury HC 3rd, Clack M. A rational for therapy of the N0 neck. Laryngoscope 1997;107:1294-315. [PubMed]
- Genden EM, Ferlito A, Bradley PJ, et al. Neck disease and distant metastases. Oral Oncol 2003;39:207-12. [PubMed]
- Mnejja M, Hammami B, Bougacha L, et al. Occult lymph node metastasis in laryngeal squamous cell carcinoma: therapeutic and prognostic impact. Eur Ann Otorhinolaryngol Head Neck Dis 2010;127:173-6. [PubMed]
- Katilmiş H, Oztürkcan S, Ozdemir I, et al. Is dissection of levels 4 and 5 justified for cN0 laryngeal and hypopharyngeal cancer? Acta Otolaryngol 2007;127:1202-6. [PubMed]
- Zhang B, Xu ZG, Tang PZ. Elective lateral neck dissection for laryngeal cancer in the clinically negative neck. J Surg Oncol 2006;93:464-7. [PubMed]
- Chone CT, Kohler HF, Magalhães R, et al. Levels II and III neck dissection for larynx cancer with N0 neck. Braz J Otorhinolaryngol (in English, Portuguese) 2012;78:59-63.
- Lim YC, Choi EC, Lee JS, et al. Is dissection of level IV absolutely necessary in elective lateral neck dissection for clinically N0 laryngeal carcinoma? Oral Oncol 2006;42:102-7. [PubMed]
- Amar A, Chedid HM, Franzi SA, et al. Neck dissection in squamous cell carcinoma of the larynx. Indication of elective contralateral neck dissection. Braz J Otorhinolaryngol (in English, Portuguese) 2012;78:7-10.
- Alpert TE, Morbidini-Gaffney S, Chung CT, et al. Radiotherapy for the clinically negative neck in supraglottic laryngeal cancer. Cancer J 2004;10:335-8. [PubMed]
- Negm H, Mosleh M, Fathy H, et al. Cytokeratin immunohistochemically detected nodal micrometastases in N0 laryngeal cancer: impact on the overall occult metastases. Eur Arch Otorhinolaryngol 2013;270:1085-92. [PubMed]
- Li Y, Li L, Wang JT, et al. Elevated content of osteopontin in plasma and tumor tissues of patients with laryngeal and hypopharyngeal carcinoma associated with metastasis and prognosis. Med Oncol 2012;29:1429-34. [PubMed]
- Elsheikh MN, Rinaldo A, Hamakawa H, et al. Importance of molecular analysis in detecting cervical lymph node metastasis in head and neck squamous cell carcinoma. Head Neck 2006;28:842-9. [PubMed]
- Hu G, Zhong S, Xiao Q, et al. Radiolocalization of Sentinel Lymph Nodes in Clinically N0 Laryngeal and Hypopharyngeal Cancers. Ann Otol Rhinol Laryngol 2011;120:345-50. [PubMed]
- Lawson G, Matar N, Nollevaux MC, et al. Reliability of Sentinel Node Technique in the Treatment of N0 Supraglottic Laryngeal Cancer. Laryngoscope 2010;120:2213-7. [PubMed]