Neoadjuvant treatment for advanced esophageal cancer: response assessment before surgery and how to predict response to chemoradiation before starting treatment
Introduction
Surgery remains the first-choice treatment for resectable esophageal cancer (1). Resection of early esophageal cancer (T1-2) usually results in long-term survival, but the prognosis is poor with esophagectomy alone for advanced stages (T3-4). For three decades, numerous trials have tested a variety of preoperative treatment strategies. Neoadjuvant radiochemotherapy or chemotherapy improved the overall survival of patients with advanced carcinomas of the esophagus by about 10% in 5 years according to current Cochrane analysis, meta-analysis, the actual prospective, randomized CROSS-trial and retrospective studies (2-5). However, the results of several trials with preoperative chemoradiation showed that only patients with a major histopathologic response will have a significant survival advantage (2,6,7). In addition, some research groups discuss the necessity of surgery for patients with complete response (CR) after preoperative chemoradiation (8). Consequently, effective methods for early and late response assessment are required in order to perform these different individualised, response-guided treatment concepts.Until now, there are several ideas for prediction of individual response to chemoradiation or chemotherapy published, e.g., endoscopy, endosonography (EUS), computed-tomography, [18F]-fluorodeoxyglucose-positron emission tomography (FDG-PET) or molecular markers. The aim of this review is to summarize the actual knowledge about the possibilities of evaluation to predict response of multimodality treatment of patients with esophageal cancer.
Response prediction or response assessment
Definition of response
There is only one valid method to evaluate the response of chemoradiation of the tumor by pathologic work-up of the resected specimen. Therefore, the gold-standard of response evaluation is the histopathologic response evaluation of the primary tumor and the resected lymph nodes (9). The WHO-classification for CR was defined as complete disappearance of all known disease. Based on the response evaluation criteria in solid tumors (RECIST), the guidelines to evaluate the response to radiotherapy, chemotherapy and chemoradiotherapy for esophageal cancers are established (10). Mostly the histopathologic regression of the primary tumor after neoadjuvant therapy is divided in four or five grades. As an example the Cologne Regression Scale is classified into four categories: grade 1, CR; grade 2, nearly CR with less than 10% vital residual tumor cells (VRTCs); grade 3, 10% to 50% VRTCs; and grade 4, greater than 50% VRTCs. Because of prognostic implications, regression grades 1 and 2 were classified as major histomorphologic response compared to grades 3 and 4, which were categorized as minor histopathologic response (6,11).
In some papers, the definition of pCR is extended to freedom from tumor in the lymph nodes. Hölscher et al. defined a combined classification of primary tumor regression and lymph node status in three grades which represent a simple and reproducible prognostic classification of the effect of neoadjuvant treatment in esophageal adenocarcinoma (12).
Response assessment
Surgery with or without neoadjuvant therapy is the therapy for choice with the best prognosis for patients with advanced esophageal cancer, providing a macroscopically and microscopically complete resection (2). Especially patients with CR of the primary tumor without lymph node metastasis had a very good prognosis with a disease specific 5-year survival rate of 68% (13). But some patients after neoadjuvant therapy are not fit enough for an extensive surgical procedure like transthoracic esophagectomy. A further question is the necessity of such a procedure after “CR”. The discussion is supported by two randomized studies comparing chemoradiation alone versus neoadjuvant chemoradiation followed by surgical therapy in patients responding to preoperative therapy (8,14). Both studies demonstrated no significantly better overall survival for patients with esophagectomy, but significantly better local disease control in the surgery group. Therefore, it could be of interest to identify the subset of patients who have achieved a major pathologic response to consider avoiding resection and on the other hand guiding the non-responders to surgical therapy. Different clinical methods for response evaluation during restaging after successful neoadjuvant therapy have been published.
Endoscopy, endosonography (EUS)
Response evaluation by endoscopy is easily performed, has very few complications, and is easily available. Lim et al. could demonstrate a significant prolonged disease-free survival after endoscopic CR 6 weeks after definitive chemoradiation for esophageal cancer (15). Adelstein et al., however, could demonstrate that endoscopic response evaluation after neoadjuvant chemoradiation had a sensitivity of 22% and a specificity of 85% only to detect pathologic CR (16). Endoscopic response evaluation is therefore by far too inaccurate to predict major histopathologic tumor regression or pathologic complete remission and is dispensable if performed for this indication. It is, therefore, important that none of the patients should be harmed by denying surgical resection if an endoscopic CR is detected.
Several groups extended response evaluation by endoscopy through taking rebiopsies to improve accuracy of response prediction. Bates et al. could show that in 7 of 17 (41%) patients with negative biopsies, tumor cells could still be detected in the resected specimens (17). In a larger series by Brown et al., 100 consecutive patients were evaluated by endoscopic rebiopsy (18). In 30 patients, rebiopsy specimens were negative for tumor cells and classified as clinical complete responders. Histopathologic evaluation of the resected specimens could demonstrate however, that a pathologic CR was present in only 15 of 30 (50%) negative rebiopsies. The results from Schneider et al. with a more extensive analysis of regression show an accuracy of 47% and, therefore, compare favorably with already published studies based on pathologic complete remission (19). A positive rebiopsy is surely the secure proof for residual tumor (positive predictive value 100%). The more important negative result, however, is too inaccurate to draw any therapeutic consequences. Sarkaria et al. performed endoscopy with biopsy for 146 patients undergoing neoadjuvant chemoradiation for esophageal cancer (20). A total of 118 patients had no tumor in the biopsy. The prognosis of patients with negative biopsy was comparable to those patients tumor in the biopsy. Therefore, all patients with negative rebiopsies should be consequently resected after neoadjuvant therapy, and for this very reason, endoscopic rebiopsy is not recommended.
EUS for classification of the cT-category before initiation of neoadjuvant therapy is known to be valuable (21). Adelstein et al. could show that clinical response evaluation by EUS according to WHO criteria has a sensitivity of 17% and a specificity of 93% (16). Similar results were reported in various studies with accuracy to determine the ypT-category after neoadjuvant therapy between 37% and 85% (19,22,23). Several problems, however, were reported using EUS for response evaluation. Downstaging is frequently seen and cannot be demonstrated by histopathology (understaging), and in patients with ypT0-and ypT1-categories overstaging is frequently present (23). Ngamruengphong et al. performed a systematic review of published information of diagnostic accuracy of EUS after completion of neoadjuvant therapy (24). The sensitivity of EUS ranged from 20% to 100% and the specificity ranged from 36% to 100%. Restaging by EUS before resection did not accurately predict pathologic stage in patients with esophageal cancer who received neoadjuvant treatment.
PET and PET/CT
FDG-PET is a valuable technique for tumor visualization, initial staging, detection of recurrent disease and radiotherapy planning in esophageal cancer (25), being even superior to anatomic imaging modalities in its ability to detect distant metastases and identify recurrent disease (26). The concept for response evaluation is that FDG-PET is performed for initial staging before neoadjuvant therapy and either after a defined number of therapy cycles or at the end of neoadjuvant treatment. All studies report on a decrease in FDG uptake comparing baseline with follow-up FDG-PET in an attempt to define a threshold, using a percentage of decrease in the standard uptake value (SUV) to separate responders from nonresponders. Systematic reviews were performed analysing the value of FDG-PET for response evaluation in the neoadjuvant therapy of patients with esophageal cancer (24,27-29). The first review was published by Westerterp et al. in 2005 comparing the diagnostic accuracy of CT, EUS, and FDG-PET for assessment of response to neoadjuvant therapy in patients with esophageal cancer (27). Rebollo Aguirre et al. included only prospective studies in their systematic review. With 8 selected articles a ranged sensitivity, specificity, positive predictive value, and negative predictive value for primary tumor response assessment by FDG-PET of 27.3% to 93.3%, 41.7% to 95.2%, 70.8% to 93.3% and 71.4% to 93.5% was calculated suggesting metabolic imaging to be the best available technique for neoadjuvant therapy response assessment in esophageal cancer (29). But all these reviews had the same problem: they included studies using different definitions of response, different cut-off values for the SUV and the time of measurement. The technique of metabolic uptake measurements in FDG-avid tumor may be another critical point. A number of factors influence the results of the SUV measurements which are subject to many sources of variability like patient size, measurement duration, plasma glucose concentration, recovery coefficients, partial volume and region of interest (ROI) selection. In fact, there was no general consensus how to perform SUV measurements and ROI size and position influence results as well as measurement of either average or maximum SUV within the ROI. Therefore, standardized methods of evaluation had to be set up (30).
Further clinical studies could not confirm the clinical relevance of response evaluation with FDG-PET after neoadjuvant therapy (31,32). The results of the prospective study from Piessen et al. showed that recurrence was not significantly correlated with the SUV values on restaging. In addition, no significant association was found between metabolic imaging and survival (P=0.106) (32). Other studies confirmed these results (33). In our attempt to address the issue of response evaluation we found considerable overlap between groups at baseline and at the end-of-neoadjuvant therapy PET, which did not allow us to separate histopathologic responders from nonresponders, regardless of histopathologic type of tumor (adenocarcinomas or squamous cell carcinomas) (34). A principle problem is that these PET scanners do not have microscopic spatial resolution which is required when the aim is to identify only small clusters of viable tumor cells defining the histopathological golden standard. Most of these studies used older imaging techniques of FDG-PET and therefore the data of the newer generation PET scanners and the combination of FDG-PET and computed tomography (CT) are of interest. New evaluation parameters were defined and seem to better correlate with histopathologic response criteria (30). But further evaluation studies are necessary.
Computed tomography (CT)
The risk of tumor progression during neoadjuvant chemoradiotherapy or chemotherapy in esophageal cancer is around 8% to 17% (2). Detection of progressive disease that alters the initial treatment strategy is of great importance, as the majority of these patients is beyond curative treatment and should be refrained from surgery. Therefore a restaging before surgical therapy is necessary. In a recently published study, the authors used small-slice (2/1.5 mm) post-CRT CT scans for adequate radiological assessment of suspect lymph nodes and/or metastatic lesions by two radiologists. Detection of progressive disease before surgery on post-CRT CT prevented futile surgery in 5 (5%) of these patients, but missed progressive disease in 4 (4%) patients (35). The study by Bruzzi et al. had distant metastases as primary outcome, comparing CT with PET-CT in the detection of distant metastasis after neoadjuvant chemoradiation. With PET-CT, it was possible to detect distant metastasis in 7 patients (8%) after neoadjuvant CRT, while CT alone detected distant metastasis in 5 (6%) of these patients. Both missed metastases were located outside the range of the routinely performed CT imaging of the chest and abdomen (36). The accuracy of multidetector-row CT (MDCT) for restaging after neoadjuvant treatment in patients with esophageal cancer was studied by Konieczny et al. (37). The authors conclude that the diagnostic accuracy of high resolution MDCT for restaging esophageal cancer and assessing the response to neoadjuvant therapy has not improved in comparison to older-generation CT.
Response prediction
Since the introduction of neoadjuvant treatment strategies, either by preoperative chemotherapy alone or in combination with external radiation for the treatment of esophageal cancer, there has been a need for response prediction before the beginning of the treatment or early response assessment identifying responders. Patients who achieve a major histopathological response have a significantly better prognosis than patients with only a minor histopathological response (2,7). Therefore, diagnostic methods for the prediction of response of induction chemotherapy are of interest for individualization of treatment concepts. Until now there are two main ideas for response prediction: measuring the response of the primary tumor by FDG-PET after an initial course of therapy or using molecular markers of the individual patient.
FDG-PET for early prediction of response
There exist several studies that scheduled the subsequent FDG-PET 7-14 days after the initiation of preoperative therapy. For example, Wieder et al. demonstrated that FDG-PET is useful for early response prediction in the course of multimodality treatment (38). In 38 patients with esophageal squamous cell cancer they analysed the therapy-induced intratumoral changes of glucose metabolism during radiochemotherapy by performing FDG-PET before and after 2 weeks of initiation of therapy. A significant decrease of intratumoral SUV was detected 2 weeks after initiation of treatment. Moreover, the decrease in SUV was significantly associated with response and prognosis. Similar results from the same institution were found for adenocarcinoma. Based on these findings the working group initiated the single-center MUNICON phase II trial to prospectively evaluate the feasibility of a FDG-PET-response-guided treatment algorithm and its potential effect on prognosis (39). The suitability of FDG-PET for response assessment has been chiefly put forward by one scientific group (38,40,41). Further studies could not confirm these favorable results. Van Heijl et al. described a clinical trial comprised serial FDG-PET before and 14 days after start of chemoradiotherapy in patients with potentially curable esophageal carcinoma. Histopathologic responders were defined as patients with no or less than 10% viable tumor cells (major response on resection specimen). FDG-PET response was measured using the SUV. From 100 included patients, 64 were histopathologic responders. The median SUV decrease 14 days after the start of therapy was 30.9% for histopathologic responders and 1.7% for non-responders (P=0.001). Using a 0% SUV decrease cutoff value, PET correctly identified 58 of 64 responders (sensitivity 91%) and 18 of 36 nonresponders (specificity 50%). The corresponding positive and negative predictive values were 76% and 75%, respectively. The authors conclude that the SUV values decrease 14 days after the start of chemoradiotherapy was significantly associated with histopathologic tumor response, but its accuracy in detecting non-responders was too low to justify the clinical use of FDG-PET for early discontinuation of neoadjuvant chemoradiotherapy in patients with potentially curable esophageal cancer (42).
Figure 1 summarizes of the published literature (41,43-46). The pooled sensitivity was 81% (95% CI: 68-92%) with a significant heterogeneity and the pooled specificity was 71% (95% CI: 64-78%). Summarizing the results of early prediction of responder after neoadjuvant chemoradiation using FDG-PET may be helpful for tailored therapy in esophageal cancer. But there are additional parameters necessary to improve the specificity of this method.
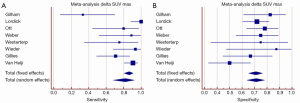
In actual research additional parameters such as entropy, size, and magnitude of local and global heterogeneous and homogeneous tumor regions were used for prediction of tumor response after neoadjuvant chemoradiation and showed promising results for response prediction (47,48). De Cobelli et al. used diffusion weighted MRI in 32 patients with gastroesophgeal cancer. The authors found a good correlation between the apparent diffusion coefficient and tumor regression (48).
Molecular markers for response prediction
A large variety of molecular markers has been reported for response prediction of neoadjuvant radiochemotherapy in pre-treatment biopsies reviewed by Fareed et al. 2009, Bain et al. 2010, Sakai et al. 2013, Kaz et al. 2014, and Okumura et al. 2014 (49-53). However, up to now none of these markers has successfully been applied for guidance of the patients to neoadjuvant treatment or direct surgery with the aim to individualize and thus improve therapy of patients with locally advanced esophageal cancer. The results detected in the “discovery cohorts” are mostly preliminary, require further validation in a larger “qualification cohort” as well as clinical translation. There are no predictive biomarkers established for esophageal cancer treatment.
Genomes, exomes and transcriptoms
Innovative techniques like next generation sequencing (NGS) and exome sequencing have been applied in the efforts to detect mutations with importance for esophageal cancer (54). These techniques provide novel biomarkers with a response predictive value, but should not end at the level of discovery.
There are several retrospective studies evaluating single nucleotide polymorphisms (SNPs) for response prediction in esophageal cancer. SNPs can alter the amino acid sequence, influence RNA splicing or translation efficacy resulting in a changed expression or activity of the proteins encoded. Response predictive impact of gene polymorphisms in pathways involved in therapy response like the DNA repair genes: ERCC1, XRCC1, ERCC2 (55), the G-protein GNAS1, and the multidrug transport: ABCB1 gene polymorphisms has been of reported (55-58).
Microarray techniques screening the whole genome for response predictive markers have largely been applied, and identified a huge variety of promising markers (59-63).
Combination of mRNA expression of several predictive markers results in better predictive value than single gene analysis (64,65). For example, in a clinical study with pretreatment biopsies of patients with an advanced esophageal cancer a single marker like Dihydropyrimidine dehydrogenase (DPD) was identified as an independent predictor associated with major response (P<0.002). Multivariate analysis of the marker combination of ERCC1, c-erbB-2 syn. Her2-neu, and DPD provided response prediction with 75.0% sensitivity, 81.0% specificity and 78.1% accuracy. Analysis of the marker panel results by Artificial Neuronal Network again showed better predictive values (64).
Thymidylate Synthase and DPD mRNA expression can also be quantified non-invasively in peripheral blood (66).
miRNome
Profiling of miRNAs, the small highly conserved post-transcriptional regulators of gene expression bears a tremendous source for detection of response predictive molecular markers reviewed by Sakai et al. and Skinner et al. (51,67). Response predictive impact of miRNAs -21, -25, 27b, -99a, -126, 133a, -b, -143, -145, and -192, has been identified (51,68). Since miRNAs are very stable their expression can simply be quantified in serum specimen. This non-invasive marker determination is advantageous for clinical use.
Proteomics
Proteome analysis has been applied for detection of predictive biomarkers by several groups (69-71). Survivin (BIRC5) protein expression has been associated with histomorphological response to neoadjuvant therapy (72). Immunhistochemical detection of ERCC1 and c-erB-B2 syn. Her2-Neu protein expression are two examples for protein markers with response predictive value (72,73).
Further perspectives: translation of research results from bench to bed will be the most crucial future challenge. We have presented a great variety of promising candidates of molecular markers for prediction of tumor response to preoperative chemoradiation. But none of these markers have been introduced in clinical practice. There are some ongoing or just finished trials; e.g., the Pancho trial—which evaluates for the first time whether the p53 genotype is qualified to select patients who will respond to certain chemotherapy and to guide cancer therapy. The results showed that the biomarker TP53 divides esophageal cancer patients into two categories with markedly different outcomes: patients with a normal TP53 marker status may experience notable benefits from neoadjuvant chemotherapy with cisplatin/fluorouracil, whereas those with a mutant TP53 marker status appear to be at risk for lack of response (74). Further results are expected from the Cologne Esophageal Response Predictive (CERP)-study, a prospective clinical study which evaluate the predictive value of ERCC1-SNP in combination with mRNA ERCC1, c-erbB-2 syn. Her2-neu, and DPD in esophageal cancer patients with chemoradiation before surgery.
Another perspective is the combination of imaging procedures and selected molecular markers. Bain et al. have reported that higher leptin protein expression were associated with lack of radiological response (75). Studies have shown that prediction with PET in clinical routine had very good sensitivity but was not valid enough to predict “nonresponse” (42). Technical innovations for better imaging of the tumor, expanded response criteria and standardized evaluation may improve the results. But a combination of optimized imaging technic and a well configured marker panel will be the future of response prediction.
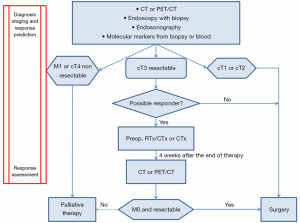
The current status of diagnosis, staging, and response prediction with its clinical implications for patients with esophageal cancer is presented in Figure 2. Four weeks after preoperative chemoradiation or chemotherapy restaging for response assessment is necessary.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Kutup A, Nentwich MF, Bollschweiler E, et al. What should be the gold standard for the surgical component in the treatment of locally advanced esophageal cancer: transthoracic versus transhiatal esophagectomy. Ann Surg 2014;260:1016-22. [PubMed]
- Hölscher AH, Bollschweiler E, Bogoevski D, et al. Prognostic impact of neoadjuvant chemoradiation in cT3 oesophageal cancer - A propensity score matched analysis. Eur J Cancer 2014;50:2950-7. [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [PubMed]
- Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol 2011;12:681-92. [PubMed]
- Ronellenfitsch U, Schwarzbach M, Hofheinz R, et al. Perioperative chemo(radio)therapy versus primary surgery for resectable adenocarcinoma of the stomach, gastroesophageal junction, and lower esophagus. Cochrane Database Syst Rev 2013;5:CD008107. [PubMed]
- Schneider PM, Baldus SE, Metzger R, et al. Histomorphologic tumor regression and lymph node metastases determine prognosis following neoadjuvant radiochemotherapy for esophageal cancer: implications for response classification. Ann Surg 2005;242:684-92. [PubMed]
- Bollschweiler E, Metzger R, Drebber U, et al. Histological type of esophageal cancer might affect response to neo-adjuvant radiochemotherapy and subsequent prognosis. Ann Oncol 2009;20:231-8. [PubMed]
- Bedenne L, Michel P, Bouché O, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol 2007;25:1160-8. [PubMed]
- Bollschweiler E, Hölscher AH, Metzger R. Histologic tumor type and the rate of complete response after neoadjuvant therapy for esophageal cancer. Future Oncol 2010;6:25-35. [PubMed]
- Japanese Society for Esophageal Diseases. Guidelines for clinical and pathologic studies on carcinoma of the esophagus, ninth edition: Preface, general principles, part I. Esophagus 2004;1:61-88.
- Baldus SE, Mönig SP, Schröder W, et al. Regression of oesophageal carcinomas after neoadjuvant radiochemotherapy: criteria of the histopathological evaluation. Pathologe 2004;25:421-7. [PubMed]
- Hölscher AH, Drebber U, Schmidt H, et al. Prognostic classification of histopathologic response to neoadjuvant therapy in esophageal adenocarcinoma. Ann Surg 2014;260:779-84;discussion 784-5. [PubMed]
- Vallböhmer D, Hölscher AH, DeMeester S, et al. A multicenter study of survival after neoadjuvant radiotherapy/chemotherapy and esophagectomy for ypT0N0M0R0 esophageal cancer. Ann Surg 2010;252:744-9. [PubMed]
- Stahl M, Stuschke M, Lehmann N, et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol 2005;23:2310-7. [PubMed]
- Lim JT, Truong PT, Berthelet E, et al. Endoscopic response predicts for survival and organ preservation after primary chemoradiotherapy for esophageal cancer. Int J Radiat Oncol Biol Phys 2003;57:1328-35. [PubMed]
- Adelstein DJ, Rice TW, Becker M, et al. Use of concurrent chemotherapy, accelerated fractionation radiation, and surgery for patients with esophageal carcinoma. Cancer 1997;80:1011-20. [PubMed]
- Bates BA, Detterbeck FC, Bernard SA, et al. Concurrent radiation therapy and chemotherapy followed by esophagectomy for localized esophageal carcinoma. J Clin Oncol 1996;14:156-63. [PubMed]
- Brown WA, Thomas J, Gotley D, et al. Use of oesophagogastroscopy to assess the response of oesophageal carcinoma to neoadjuvant therapy. Br J Surg 2004;91:199-204. [PubMed]
- Schneider PM, Metzger R, Schaefer H, et al. Response evaluation by endoscopy, rebiopsy, and endoscopic ultrasound does not accurately predict histopathologic regression after neoadjuvant chemoradiation for esophageal cancer. Ann Surg 2008;248:902-8. [PubMed]
- Sarkaria IS, Rizk NP, Bains MS, et al. Post-treatment endoscopic biopsy is a poor-predictor of pathologic response in patients undergoing chemoradiation therapy for esophageal cancer. Ann Surg 2009;249:764-7. [PubMed]
- Qumseya BJ, Brown J, Abraham M, et al. Diagnostic performance of EUS in predicting advanced cancer among patients with Barrett's esophagus and high-grade dysplasia/early adenocarcinoma: systematic review and meta-analysis. Gastrointest Endosc 2015;81:865-874.e2.
- Griffin JM, Reed CE, Denlinger CE. Utility of restaging endoscopic ultrasound after neoadjuvant therapy for esophageal cancer. Ann Thorac Surg 2012;93:1855-9;discussion 1860.
- Misra S, Choi M, Livingstone AS, et al. The role of endoscopic ultrasound in assessing tumor response and staging after neoadjuvant chemotherapy for esophageal cancer. Surg Endosc 2012;26:518-22. [PubMed]
- Ngamruengphong S, Sharma VK, Nguyen B, et al. Assessment of response to neoadjuvant therapy in esophageal cancer: an updated systematic review of diagnostic accuracy of endoscopic ultrasonography and fluorodeoxyglucose positron emission tomography. Dis Esophagus 2010;23:216-31. [PubMed]
- Bombardieri E. The added value of metabolic imaging with FDG-PET in oesophageal cancer: prognostic role and prediction of response to treatment. Eur J Nucl Med Mol Imaging 2006;33:753-8. [PubMed]
- Skehan SJ, Brown AL, Thompson M, et al. Imaging features of primary and recurrent esophageal cancer at FDG PET. Radiographics 2000;20:713-23. [PubMed]
- Westerterp M, van Westreenen HL, Reitsma JB, et al. Esophageal cancer: CT, endoscopic US, and FDG PET for assessment of response to neoadjuvant therapy--systematic review. Radiology 2005;236:841-51. [PubMed]
- Suttie SA, Welch AE, Park KG. Positron emission tomography for monitoring response to neoadjuvant therapy in patients with oesophageal and gastro-oesophageal junction carcinoma. Eur J Surg Oncol 2009;35:1019-29. [PubMed]
- Rebollo Aguirre AC, Ramos-Font C, Villegas Portero R, et al. 18F-fluorodeoxiglucose positron emission tomography for the evaluation of neoadjuvant therapy response in esophageal cancer: systematic review of the literature. Ann Surg 2009;250:247-54. [PubMed]
- Metser U, Rashidi F, Moshonov H, et al. (18)F-FDG-PET/CT in assessing response to neoadjuvant chemoradiotherapy for potentially resectable locally advanced esophageal cancer. Ann Nucl Med 2014;28:295-303. [PubMed]
- Vallböhmer D, Hölscher AH, Dietlein M, et al. [18F]-Fluorodeoxyglucose-positron emission tomography for the assessment of histopathologic response and prognosis after completion of neoadjuvant chemoradiation in esophageal cancer. Ann Surg 2009;250:888-94. [PubMed]
- Piessen G, Petyt G, Duhamel A, et al. Ineffectiveness of 18F-fluorodeoxyglucose positron emission tomography in the evaluation of tumor response after completion of neoadjuvant chemoradiation in esophageal cancer. Ann Surg 2013;258:66-76. [PubMed]
- Cheedella NK, Suzuki A, Xiao L, et al. Association between clinical complete response and pathological complete response after preoperative chemoradiation in patients with gastroesophageal cancer: analysis in a large cohort. Ann Oncol 2013;24:1262-6. [PubMed]
- Schmidt M, Bollschweiler E, Dietlein M, et al. Mean and maximum standardized uptake values in [18F]FDG-PET for assessment of histopathological response in oesophageal squamous cell carcinoma or adenocarcinoma after radiochemotherapy. Eur J Nucl Med Mol Imaging 2009;36:735-44. [PubMed]
- Hulshoff JB, Smit JK, van der Jagt EJ, et al. Evaluation of progression prior to surgery after neoadjuvant chemoradiotherapy with computed tomography in esophageal cancer patients. Am J Surg 2014;208:73-9. [PubMed]
- Bruzzi JF, Swisher SG, Truong MT, et al. Detection of interval distant metastases: clinical utility of integrated CT-PET imaging in patients with esophageal carcinoma after neoadjuvant therapy. Cancer 2007;109:125-34. [PubMed]
- Konieczny A, Meyer P, Schnider A, et al. Accuracy of multidetector-row CT for restaging after neoadjuvant treatment in patients with oesophageal cancer. Eur Radiol 2013;23:2492-502. [PubMed]
- Wieder HA, Ott K, Lordick F, et al. Prediction of tumor response by FDG-PET: comparison of the accuracy of single and sequential studies in patients with adenocarcinomas of the esophagogastric junction. Eur J Nucl Med Mol Imaging 2007;34:1925-32. [PubMed]
- Lordick F, Ott K, Krause BJ, et al. PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: the MUNICON phase II trial. Lancet Oncol 2007;8:797-805. [PubMed]
- Wieder HA, Brücher BL, Zimmermann F, et al. Time course of tumor metabolic activity during chemoradiotherapy of esophageal squamous cell carcinoma and response to treatment. J Clin Oncol 2004;22:900-8. [PubMed]
- Ott K, Weber W, Siewert JR. The importance of PET in the diagnosis and response evaluation of esophageal cancer. Dis Esophagus 2006;19:433-42. [PubMed]
- van Heijl M, Omloo JM, van Berge Henegouwen MI, et al. Fluorodeoxyglucose positron emission tomography for evaluating early response during neoadjuvant chemoradiotherapy in patients with potentially curable esophageal cancer. Ann Surg 2011;253:56-63. [PubMed]
- Gillham CM, Lucey JA, Keogan M, et al. (18)FDG uptake during induction chemoradiation for oesophageal cancer fails to predict histomorphological tumour response. Br J Cancer 2006;95:1174-9. [PubMed]
- Weber WA, Ott K, Becker K, et al. Prediction of response to preoperative chemotherapy in adenocarcinomas of the esophagogastric junction by metabolic imaging. J Clin Oncol 2001;19:3058-65. [PubMed]
- Westerterp M, Omloo JM, Sloof GW, et al. Monitoring of response to pre-operative chemoradiation in combination with hyperthermia in oesophageal cancer by FDG-PET. Int J Hyperthermia 2006;22:149-60. [PubMed]
- Gillies RS, Middleton MR, Blesing C, et al. Metabolic response at repeat PET/CT predicts pathological response to neoadjuvant chemotherapy in oesophageal cancer. Eur Radiol 2012;22:2035-43. [PubMed]
- Tixier F, Le Rest CC, Hatt M, et al. Intratumor heterogeneity characterized by textural features on baseline 18F-FDG PET images predicts response to concomitant radiochemotherapy in esophageal cancer. J Nucl Med 2011;52:369-78. [PubMed]
- De Cobelli F, Giganti F, Orsenigo E, et al. Apparent diffusion coefficient modifications in assessing gastro-oesophageal cancer response to neoadjuvant treatment: comparison with tumour regression grade at histology. Eur Radiol 2013;23:2165-74. [PubMed]
- Fareed KR, Kaye P, Soomro IN, et al. Biomarkers of response to therapy in oesophago-gastric cancer. Gut 2009;58:127-43. [PubMed]
- Bain GH, Petty RD. Predicting response to treatment in gastroesophageal junction adenocarcinomas: combining clinical, imaging, and molecular biomarkers. Oncologist 2010;15:270-84. [PubMed]
- Sakai NS, Samia-Aly E, Barbera M, et al. A review of the current understanding and clinical utility of miRNAs in esophageal cancer. Semin Cancer Biol 2013;23:512-21. [PubMed]
- Kaz AM, Grady WM. Epigenetic biomarkers in esophageal cancer. Cancer Lett 2014;342:193-9. [PubMed]
- Okumura H, Uchikado Y, Setoyama T, et al. Biomarkers for predicting the response of esophageal squamous cell carcinoma to neoadjuvant chemoradiation therapy. Surg Today 2014;44:421-8. [PubMed]
- Dulak AM, Stojanov P, Peng S, et al. Exome and whole-genome sequencing of esophageal adenocarcinoma identifies recurrent driver events and mutational complexity. Nat Genet 2013;45:478-86. [PubMed]
- Warnecke-Eberz U, Vallböhmer D, Alakus H, et al. ERCC1 and XRCC1 gene polymorphisms predict response to neoadjuvant radiochemotherapy in esophageal cancer. J Gastrointest Surg 2009;13:1411-21. [PubMed]
- Alakus H, Bollschweiler E, Hölscher AH, et al. Homozygous GNAS 393C-allele carriers with locally advanced esophageal cancer fail to benefit from platinum-based preoperative chemoradiotherapy. Ann Surg Oncol 2014;21:4375-82. [PubMed]
- Alakus H, Warnecke-Eberz U, Bollschweiler E, et al. GNAS1 T393C polymorphism is associated with histopathological response to neoadjuvant radiochemotherapy in esophageal cancer. Pharmacogenomics J 2009;9:202-7. [PubMed]
- Narumiya K, Metzger R, Bollschweiler E, et al. Impact of ABCB1 C3435T polymorphism on lymph node regression in multimodality treatment of locally advanced esophageal cancer. Pharmacogenomics 2011;12:205-14. [PubMed]
- Lu P, Qiao J, He W, et al. Genome-wide gene expression profile analyses identify CTTN as a potential prognostic marker in esophageal cancer. PLoS One 2014;9:e88918. [PubMed]
- Metzger R, Heukamp L, Drebber U, et al. CUL2 and STK11 as novel response-predictive genes for neoadjuvant radiochemotherapy in esophageal cancer. Pharmacogenomics 2010;11:1105-13. [PubMed]
- Duong C, Greenawalt DM, Kowalczyk A, et al. Pretreatment gene expression profiles can be used to predict response to neoadjuvant chemoradiotherapy in esophageal cancer. Ann Surg Oncol 2007;14:3602-9. [PubMed]
- Schauer M, Janssen KP, Rimkus C, et al. Microarray-based response prediction in esophageal adenocarcinoma. Clin Cancer Res 2010;16:330-7. [PubMed]
- Ashida A, Boku N, Aoyagi K, et al. Expression profiling of esophageal squamous cell carcinoma patients treated with definitive chemoradiotherapy: clinical implications. Int J Oncol 2006;28:1345-52. [PubMed]
- Warnecke-Eberz U, Metzger R, Bollschweiler E, et al. TaqMan low-density arrays and analysis by artificial neuronal networks predict response to neoadjuvant chemoradiation in esophageal cancer. Pharmacogenomics 2010;11:55-64. [PubMed]
- Rumiato E, Cavallin F, Boldrin E, et al. ERCC1 C8092A (rs3212986) polymorphism as a predictive marker in esophageal cancer patients treated with cisplatin/5-FU-based neoadjuvant therapy. Pharmacogenet Genomics 2013;23:597-604. [PubMed]
- Brabender J, Metzger R, Vallböhmer D, et al. Roles of thymidylate synthase and dihydropyrimidine dehydrogenase expression in blood as predictors of response to multimodal therapy in esophageal cancer. Surgery 2012;151:306-12. [PubMed]
- Skinner HD, Lee JH, Bhutani MS, et al. A validated miRNA profile predicts response to therapy in esophageal adenocarcinoma. Cancer 2014;120:3635-41. [PubMed]
- Odenthal M, Bollschweiler E, Grimminger PP, et al. MicroRNA profiling in locally advanced esophageal cancer indicates a high potential of miR-192 in prediction of multimodality therapy response. Int J Cancer 2013;133:2454-63. [PubMed]
- Kelly P, Appleyard V, Murray K, et al. Detection of oesophageal cancer biomarkers by plasma proteomic profiling of human cell line xenografts in response to chemotherapy. Br J Cancer 2010;103:232-8. [PubMed]
- Herrmann K, Walch A, Balluff B, et al. Proteomic and metabolic prediction of response to therapy in gastrointestinal cancers. Nat Clin Pract Gastroenterol Hepatol 2009;6:170-83. [PubMed]
- Izzo JG, Luthra R, Sims-Mourtada J, et al. Emerging molecular targets in esophageal cancers. Gastrointest Cancer Res 2007;1:S3-6. [PubMed]
- Vallböhmer D, Kuhn E, Warnecke-Eberz U, et al. Failure in downregulation of intratumoral survivin expression following neoadjuvant chemoradiation in esophageal cancer. Pharmacogenomics 2008;9:681-90. [PubMed]
- Miyazono F, Metzger R, Warnecke-Eberz U, et al. Quantitative c-erbB-2 but not c-erbB-1 mRNA expression is a promising marker to predict minor histopathologic response to neoadjuvant radiochemotherapy in oesophageal cancer. Br J Cancer 2004;91:666-72. [PubMed]
- Kandioler D, Schoppmann SF, Zwrtek R, et al. The biomarker TP53 divides patients with neoadjuvantly treated esophageal cancer into 2 subgroups with markedly different outcomes. A p53 Research Group study. J Thorac Cardiovasc Surg 2014;148:2280-6. [PubMed]
- Bain GH, Collie-Duguid E, Murray GI, et al. Tumour expression of leptin is associated with chemotherapy resistance and therapy-independent prognosis in gastro-oesophageal adenocarcinomas. Br J Cancer 2014;110:1525-34. [PubMed]


