Impact of chronic exposure to bevacizumab on EpCAM-based detection of circulating tumor cells
Introduction
In the last few years, increasing evidence suggests that circulating tumor cells (CTCs) play a principal role in dissemination from primary tumors in the generation of distant metastases (1).
CTCs can be isolated from blood in a non-invasive approach and can be used to follow patients over time since these cells can provide significant information for a better understanding of tumor biology and tumor dissemination (2). Several methods have been developed to detect and isolate CTCs from the peripheral blood of patients with cancer (3). To date, CellSearch® System (Janssen Diagnostics, LLC, Raritan, NJ, USA) is the only Food and Drug Administration (FDA) approved method used to obtain prognostic information through CTC counts. The system performs automated immunomagnetic epithelial cell adhesion molecule (EpCAM) based enrichment followed by cytokeratins (CK) staining of cells in blood samples (4). In the last years, evidence is emerging regarding a possible drug-related impairment in the predictive value of CTCs during treatment. Specifically, data from literature show that CTC count through CellSearch® in metastatic breast and colorectal cancer (CRC) patients treated with bevacizumab (BEV) retains a significant prognostic value at baseline but lacks predictive significance as an early surrogate marker of response when assessed under treatment (5,6).
It is also hypothesized that BEV (I) may generate in the primary tumor a selected population of cells undergoing epithelial to mesenchymal transition (EMT) (6); this process evokes a change from a polarized epithelial phenotype, in which cells are adherent to the basement membrane and express classical epithelial markers including EpCAM, to a mesenchymal state in which cell-cell contact is lost and mesenchymal markers are expressed; markers of EMT program include downregulation of CK, E-cadherin and β-catenin and upregulation of N-cadherin and vimentin (7); (II) may act directly on the tumor cells (5), in same way modifying the EpCAM expression through not still established mechanisms. Therefore a different EpCAM isoform expression might be responsible for failure of CellSearch® System to detect CTCs, since isolation and detection of CTC with immunomagnetic enrichment methods is critically dependent on the used EpCAM clone (8).
In order to verify these hypotheses, in the present preliminary study we sought to investigate whether chronic exposure to BEV at clinically relevant doses (9) may alter proteins involved in EMT (CK, E-cadherin, N-cadherin, β-catenin, vimentin) or alternatively may induce EpCAM modifications in human CRC cell lines in vitro. The ability of CellSearch® to capture BEV-treated cells has been also investigated.
Materials and methods
Antibodies
Antibodies for western blot and immunofluorescence analysis were as follows: monoclonal mouse anti-EpCAM, monoclonal mouse anti-vimentin, monoclonal mouse anti-β-catenin, polyclonal rabbit anti-N-cadherin, monoclonal mouse anti-E-cadherin (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) and monoclonal mouse anti-cytokeratin 8, 18, 19 (Abcam, Cambridge, MA, USA).
Cell lines and culture conditions
WiDr human CRC cell line was obtained from Interlab Cell Line Collection (Genova, GE, Italy). Cell line was cultured in minimal essential medium (MEM) supplemented with 10% fetal bovine serum (FBS) (SIGMA-ALDRICH, St. Louis, MO, USA), penicillin-streptomycin, L-glutamine and non essential amino acids (EuroClone S.P.A., Pero, MI, Italy) at 37 °C in 5% CO2 and 95% air.
The human CRC cell line WiDr was exposed to a clinically relevant dose of BEV (250 μg/mL) for 3 months in vitro. Results from all in vitro studies were confirmed in at least three independent experiments to verify results. All the experiments were performed when cells reached 50-60% confluence.
Untreated WiDr cells were used as a control.
Protein extraction and western blot
Cells were washed twice with cold PBS, collected and centrifuged at 400 g for 10 min. Cell pellets were solubilised in 10 mM Tris-HCl pH 7.6, 160 mM NaCl, 1 mM EGTA, 1% deoxycholic acid, 1% Triton, 0.1% SDS and one complete mini-protease inhibitor cocktail tablet (SIGMA Chemicals Company, St. Louis. MO, USA), incubated on ice for 30’ and centrifuged at 12,000 g for 30 min; the supernatant was then collected.
Protein extracts (30 µg) were incubated at 100 °C for 10 min and separated on 4-12% SDS-PAGE gel, blotted onto PVDF membrane (GE Healthcare, Milano, Italy) and probed with primary antibodies overnight at 4 °C.
The next day, they were washed three times for 10 min with TBS and 0.1% Tween-20 and re-probed with the appropriate secondary antibody for 1 h at room temperature.
After incubation and three washes, immunoreactive bands were visualized by enhanced chemoluminescence (PerkinElmer, Waltham, MA, USA).
Mouse anti-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA) served as a protein loading control.
Results were confirmed in at least three independent experiments to verify results.
Immunofluorescence
Cells, grown on coverslips, untreated and treated as described above, were fixed with 4% paraformaldehyde in PBS for 30 min, washed in 0.1 M glycine for 20 min, and permeabilized in 0.1% Triton X-100 for additional 5 min.
After blocking for 5 min with 1% BSA cells were incubated with the following primary antibodies and visualized using FITC-conjugated goat anti-mouse IgG fluorescein isothiocyanate-conjugated (Alexa 488, Life Technologies) for additional 30 min at room temperature. The nuclei were stained with 4,6-diamido-2-phenylindole (DAPI, Sigma-Aldrich, Milan, Italy).
Cells were analyzed using an Apotome Axio Observer Z1 inverted microscope (Zeiss, Oberkochen, Germany), equipped with an AxioCam MRM Rev.3 at 40× magnification. Image analysis was performed using Adobe Photoshop.
All experiments included negative controls with secondary antibody alone.
Results were confirmed in at least three independent experiments to verify results.
WiDr detection by CellSearch® System
To evaluate the impact of chronic exposure to BEV on CTC enumeration by CellSearch®, untreated and BEV-treated colon cancer cell (WiDr) were spiked into blood samples from a healthy volunteer. Four experiments have been performed: before treatment (time 0), and in course of treatment (1, 2, 3 months). A defined number (50 individual cells) was collected by pipetting under microscopic control and directly transferred into the blood sample which was then processed by CellSearch®.
Results were confirmed in at least three independent experiments to verify results.
Results
To study the effects of chronic BEV exposure on CRC cells, the expression of epithelial and mesenchymal markers were determined by immunofluorescence and western blot in untreated and BEV-treated cell lines after 1, 2 and 3 months of exposure. At 1 and 2 months after treatment no differences were observed between untreated and treated cells, neither at immunofluorescence nor at western blot analysis (data not shown).
At three months of treatment, immunofluorescence analysis showed that BEV neither altered the expression of vimentin and N-cadherin (both absent in untreated and treated cells), nor that of E-cadherin, EpCAM and β-catenin (unchanged in untreated and treated cells), but induced a weak decrease in the expression of CK (Figure 1).
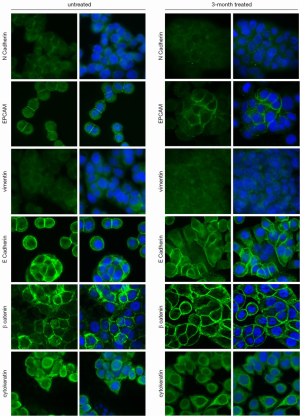
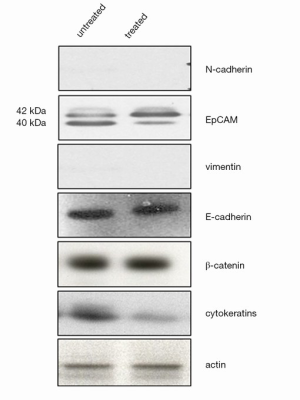
Western blot analysis confirmed that E-cadherin and β-catenin levels did not change in treated cells compared to untreated cells, whereas CK expression decreased in treated cells. Vimentin and N-cadherin were also found undetectable in both untreated and treated cell lines. Interestingly, it revealed in treated WiDr cell lines a significant increase of EpCAM 42 kDa isoform expression and a concomitant decrease of EpCAM 40 kDa isoform, compared to controls (Figure 2).
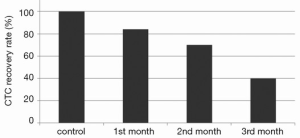
To evaluate the impact of EpCAM variant isoforms expression on CTC enumeration by CellSearch®, 50 untreated cells and 50 BEV treated colon cancer cells (1, 2, 3 months) were spiked into 7.5 mL of blood from an healthy donor and enumerated by CellSearch®. The recovery rate of cells through CellSearch® was gradually reduced in course of treatment with BEV, being 84%, 70% and 40% at 1, 2 and 3 months, respectively (Figure 3).
Discussion
During the last five years, it has been demonstrated that BEV combined with chemotherapy may modify the predictive value of CTC during treatment. In an exploratory prospective substudy reported by Bidard et al. in 2010 (5), CTCs were screened by CellSearch® assay in patients who received BEV combined with chemotherapy as first-line treatment of metastatic breast cancer. CTCs were found of prognostic significance for time to progression (TTP) and predictive of tumor response at baseline, but not 6 weeks after the start of the treatment. Authors hypothesized that the lack of CTC significance during treatment may be directly related to the systematic use of BEV, probably due to its direct anti-CTC effect. Alternatively, they also suggested that the use of BEV may change the intravasation abilities of cancer cells, due to its effect on the vessels endothelium.
Furthermore, in 2011 our group investigated the predictive value of CTC count in patients with metastatic CRC treated with a first-line chemotherapy plus BEV compared with those treated with chemotherapy plus cetuximab. CTC count was carried out by CellSearch® assay. A total of 56% of patients treated with BEV with 0 CTC at follow-up evaluation had a progressive disease (PD), while in the group treated with cetuximab, patients with persistent high CTC count were found in PD and patients with decreased number of CTC were found with stable disease or partial response. We hypothesized that BEV, through the inhibition of tumor angiogenesis and consequently the induction of hypoxia, may generate in the primary tumor a selected population of cells undergoing EMT, which loose cell-cell contact, become motile and acquire mesenchymal features (6).
To verify this hypothesis, we now sought to investigate whether BEV may exert direct effects on CRC cells in vitro attributable to the generation of EMT phenotype. To this purpose, and due to the difficulty to generate CTC cell lines, we used the human colon cancer WiDr cell line to be exposed to a clinically relevant dose of BEV.
We failed to find any morphological evidence of EMT in vitro, probably due to the necessity of contact with endothelial surface or organ systems in vivo, as previously suggested (10). Nevertheless, we found that BEV induces a decreased expression of CK, strongly evident at 3 months of treatment, which may also have impaired CTC enumeration through CellSearch®. In congruence with this, Gökmen-Polar et al. recently demonstrated that in breast cancer BEV treatment may result in the deregulation of one or more high molecular weight/basal keratins (11). We then sought to investigate whether BEV may induce some alteration in EpCAM expression, which might account for the lack of predictive significance of CellSearch® in colon cancer patients treated with antiangiogenic therapy.
We showed for the first time that BEV induces a decrease of EpCAM 40 kDa isoform expression and an increase of EpCAM 42 kDa isoform expression. We have no explanation for the appearance of the 42 kDa EpCAM isoform, although we may speculate that one of these would be glycosylation. In fact, it has been reported that most carcinoma cell lines produce multiple EpCAM isoforms of 38, 40, 42 kDa as a result of differential glycosylation of the polypeptide backbone. Indeed, the sequence of EpCAM molecule predicts three potential N-linked glycosylation sites (12), and the attached carbohydrates likely contain mannose oligosaccharide chains (13). Unfortunately, the EpCAM antibody used in the CellSearch® capture system is not known, so that our hypothesis remains speculative.
To evaluate the impact of EpCAM variant isoforms expression on CTC enumeration by CellSearch®, we have spiked untreated and BEV treated colon cancer cell into blood samples which were then enumerated. The recovery rate of cells through CellSearch® was gradually reduced in course of treatment with BEV, being only 40% after 3 months, concomitant with the strongest decrease of EpCAM 40 kDa isoform as demonstrated by Western blot analysis. Thus, it is conceivable that BEV may impair CTC recovery by CellSearch® not only through the induction of EMT, as we had previously hypothesized, but through a modulation of EpCAM isoforms. These hypotheses are congruent with the results of the study by Antolovic et al., who for the first time showed that isolation and detection of CTC with immunomagnetic enrichment methods is critically dependent on the used EpCAM clone. The dissimilar capture of CTC by two different mAbs in their analysis was explained by various expression of the EpCAM molecule among the examined patients (8).
BEV-dependent modification of EpCAM and CK suggests that EpCAM-based enrichment techniques may lead to the failure to detect CTC in BEV treated patients. Our findings may explain clinical results where low CTC numbers have been reported even in patients with late metastatic cancers.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Nguyen DX, Bos PD, Massagué J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer 2009;9:274-84. [PubMed]
- Lianidou ES, Strati A, Markou A. Circulating tumor cells as promising novel biomarkers in solid cancers. Crit Rev Clin Lab Sci 2014;51:160-71. [PubMed]
- Alix-Panabières C, Pantel K. Technologies for detection of circulating tumor cells: facts and vision. Lab Chip 2014;14:57-62. [PubMed]
- Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 2004;351:781-91. [PubMed]
- Bidard FC, Mathiot C, Degeorges A, et al. Clinical value of circulating endothelial cells and circulating tumor cells in metastatic breast cancer patients treated first line with bevacizumab and chemotherapy. Ann Oncol 2010;21:1765-71. [PubMed]
- Gazzaniga P, Raimondi C, Gradilone A, et al. Circulating tumor cells, colon cancer and bevacizumab: the meaning of zero. Ann Oncol 2011;22:1929-30. [PubMed]
- Iwatsuki M, Mimori K, Yokobori T, et al. Epithelial-mesenchymal transition in cancer development and its clinical significance. Cancer Sci 2010;101:293-9. [PubMed]
- Antolovic D, Galindo L, Carstens A, et al. Heterogeneous detection of circulating tumor cells in patients with colorectal cancer by immunomagnetic enrichment using different EpCAM-specific antibodies. BMC Biotechnol 2010;10:35. [PubMed]
- Fan F, Samuel S, Gaur P, et al. Chronic exposure of colorectal cancer cells to bevacizumab promotes compensatory pathways that mediate tumour cell migration. Br J Cancer 2011;104:1270-7. [PubMed]
- Gorges TM, Tinhofer I, Drosch M, et al. Circulating tumour cells escape from EpCAM-based detection due to epithelial-to-mesenchymal transition. BMC Cancer 2012;12:178. [PubMed]
- Gökmen-Polar Y, Goswami CP, Toroni RA, et al. Gene Expression Analysis Reveals Distinct Pathways of Resistance to Bevacizumab in Xenograft Models of Human ER-Positive Breast Cancer. J Cancer 2014;5:633-45. [PubMed]
- Balzar M, Winter MJ, de Boer CJ, et al. The biology of the 17-1A antigen (Ep-CAM). J Mol Med (Berl) 1999;77:699-712. [PubMed]
- Durbin H, Rodrigues N, Bodmer WF. Further characterization, isolation and identification of the epithelial cell-surface antigen defined by monoclonal antibody AUA1. Int J Cancer 1990;45:562-5. [PubMed]


