Prognostic effect analysis of molecular subtype on young breast cancer patients
Introduction
Currently, breast cancer is the most common cancer in Chinese women. This trend is also observed in many other countries. And 12.2% of newly diagnosed breast cancers and 9.6% of deaths due to breast cancer worldwide are from cancer patients in China (1). In the United States, women younger than 40 years old account for 6.6% of breast cancer cases (2). According to International Agency for Research on Cancer, breast cancer cases in women less than 40 years old accounted for 12.56% of all breast cancers in China in 2008, much higher than the proportion in western countries (3). It is commonly recognized that young breast cancer patients are likely to have higher degree of tumor malignancy, higher rate of relapse within an early time period, and worse prognosis (4-6). It is of great clinical importance and social value to investigate biological indicators for breast cancer prognosis, especially for young patients in China. The molecular subtypes of breast cancer were introduced in 2011 (7). Breast cancer of different molecular subtypes has different biological behavior, suggesting that molecular subtype has important value in comprehensive evaluation of prognosis. There is relatively little literature in China focusing on the prognostic effect of molecular subtypes on young breast cancer patients. This study therefore performed a retrospective analysis of 187 breast cancer patients less than 40 years old treated in Obstetrics and Gynecology Hospital of Fudan University between June 2005 and June 2011.
Patients and methods
Patients
A retrospective study was conducted, in which breast cancer patients less than 40 years old treated in Obstetrics and Gynecology Hospital of Fudan University between June 2005 and June 2011 were enrolled. Inclusion criteria were: (I) pathologic type was breast invasive ductal carcinoma; (II) no evidence of distant metastasis such as bone, liver, lung, brain and so on; (III) underwent mastectomy or breast conserving surgery (BCT); (IV) estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER-2) and Ki67 were evaluated by immunohistochemistry staining. HER-2(3+) was defined as HER-2 overexpression, HER-2(−) or HER-2(+) was defined as HER-2 negative. Fluorescence in situ hybridization (FISH) was needed in cases of HER-2(2+). According to the 2013 St Gallen expert consensus (8), 4 molecular subtypes are defined as: (i) Luminal A, all of: ER and PR positive, HER-2 negative, Ki-67 level <14%; (ii) Luminal B, ER positive, HER-2 negative, and at least one of: Ki-67 >14%, PR negative; or ER positive, HER-2 overexpression, any Ki-67, any PR; (iii) HER-2, HER-2 overexpression, ER and PR negative; and (iv) triple negative breast cancer (TNBC), ER and PR negative, HER-2 negative; (V) intact follow-up records in the hospital. Altogether 187 patients were enrolled in this study and 2 patients were excluded due to lost to follow-up. The 187 patients were then classified into the 4 molecular subtypes retrospectively according to the 2013 St Gallen expert consensus.
Follow-up
The follow-up duration was calculated from the date of diagnosis until the date of death or last contact. Disease-free survival (DFS) was the time between diagnosis and confirmation of disease relapse. Overall survival (OS) was the time between diagnosis and death as a result of recurrence events. The follow-up deadline was June 2014.
Statistical analysis
In this study, the clinical-pathological characteristics were analyzed according to molecular subtypes, such as age, tumor size, histological grade, lymph node status, lymphovascular invasion, TMN stage, surgery type, chemotherapy, radiation therapy, endocrine therapy, etc. The Student’s t test and χ2 test (Pearson statistic) were used to determine the differences in clinical-pathological characteristics between different molecular subtypes of patients. The 5-year DFS and OS were analyzed. Factors such as tumor size (T≤2 cm, T>2 cm), lymph node status, histological grade, lymphovascular invasion, TMN stage, surgery type, chemotherapy, radiation therapy and molecular subtype were taken into univariate analysis to show the association with DFS and OS. Survival estimates were computed using the Kaplan-Meier method and differences between survival times were assessed by means of the Log rank test. Factors with statistical significance in univariate analysis were taken into multivariate analysis to show the independent prognostic factors of DFS and OS. Multivariate analyses were carried out using Cox’s proportional hazards model. All statistical analyses were carried out using the SPSS (version 19.0) software package (SPSS Inc., Chicago, IL, USA). P<0.05 was considered statistically significant.
Results
Clinical-pathological characteristics of young breast cancer patients
A total of 187 young breast cancer patients were premenopausal with an average age of 35.36±3.88 years old. The mean tumor size was 2.43±1.53 cm; 81 cases had lymph node metastasis (43.3%), 126 cases had lymphovascular invasion (67.4%), and 125 cases had histological grade III (66.8%) disease. Of the 187 cases, 135 underwent mastectomy whereas 52 breast-conserving surgery.
These 187 cases were retrospectively divided into 4 molecular subtypes according to the 2013 St Gallen expert consensus: 27 cases in Luminal A subtype (14.4%); 99 cases in Luminal B subtype (52.9%); 29 cases in HER-2 subtype (15.5%); and 32 cases in TNBC subtype (17.1%). Clinical-pathological characteristics such as age, histological grade, TMN stage, lymphovascular invasion, surgery type or radiation therapy, did not display a difference among the 4 molecular subtypes in this study (Table 1).
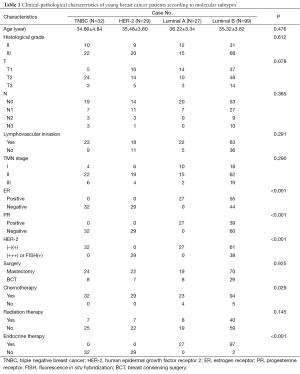
Full table
Nine cases did not receive chemotherapy (4 cases in Luminal A and 5 cases in Luminal B). These cases were all in TMN stage I with a tumor size less than 2 cm, negative lymph nodes, histological grade II, high ER level, HER-2 negativity and negative lymphovascular invasion. The other cases received adjuvant chemotherapy or neoadjuvant chemotherapy. Cases with 4 or more lymph node metastases, a tumor size over 5 cm, or receiving BCT routinely underwent adjuvant radiation therapy after surgery. Cases that were ER or PR positive received endocrine therapy. Two cases ceased endocrine therapy because of intolerance of adverse effect of the drugs. Cases with HER-2 overexpression were strongly recommended trastuzumab. Eighteen cases in HER-2 subtype received trastuzumab, while 10 cases in Luminal B subtype, in which 38 cases had HER-2 overexpression, received trastuzumab.
Survival analysis of young breast cancer patients and prognostic factors of survival
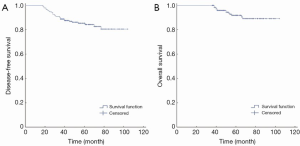
Within a median follow-up period of 61 months (36-104 months), tumor relapse occurred in 29 cases, among which 13 cases died (Table 2). The 5-year DFS was 84% and the 5-year OS was 92% (Figure 1).

Full table
At univariate analysis, lymph node status (P=0.038) and molecular subtype (P=0.044) were significantly associated with DFS (Figure 2A,B). When the molecular subtypes were divided into TNBC and non-TNBC, the association was still significant between molecular subtype and DFS (P=0.038) (Figure 2C). Lymph node status was significantly associated with OS (P=0.015), whereas molecular subtype was not statistically significantly associated with OS (P=0.060) (Figure 3A,B). When the molecular subtypes were divided into TNBC and non-TNBC, the molecular subtype was statistically significantly associated with OS (P=0.023) (Figure 3C).


At multivariate analysis, lymph node status (P=0.041) and molecular subtype (P=0.037) were both independent prognostic factors for DFS (Table 3). When molecular subtypes were divided into TNBC and non-TNBC, the lymph node status (P=0.037) and TNBC subtype (P=0.048) were both independent prognostic factors for OS (Table 4).
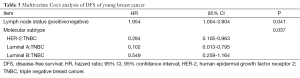
Full table

Full table
Discussion
Whether age is an independent risk factor for breast cancer survival is controversial. Han et al. supported that young age (<35 years old) is an independent risk factor for relapse in operable breast cancer patients through multivariate analysis of OS (9). However, other scholars identified that age was not significantly related to mortality from breast cancer when accounting for all prognostic variables (10). Although there is no perspective study supporting young age as an independent prognostic factor, it can be regarded as a risk predictor for survival. Age should be considered, in association with other pathological and biological factors, in the treatment of breast cancer, so that young breast cancer patients can receive more effective therapeutic regimens.
Molecular subtypes of breast cancer were introduced to reflect the biology of tumors and marked differences in patient prognosis (11,12). The distribution of molecular subtypes is somewhat different among young patients and their older counterparts. Compared with older women, young women had higher proportions of hormone receptor (HR)(+)/HER-2(+), triple negative and HR(−)/HER-2(+) breast cancer (13). Furthermore, there may be differences in prognosis even for the same subtype. For example, Luminal B tumors among young women, when compared with the older group, demonstrated more aggressive features (14) and had worse outcomes (15). An Italian, institution-based study found worse survival in women <35 years of age compared with older women (35 to 50 years of age) for triple-negative, Luminal B, and HER-2-positive breast cancer (15). Recently, molecular subtypes showed a prognostic association with outcome in patients <65 years of age with regards to the relapse free period (RFP) (P=0.01) and relative survival (RS) (P<0.001). However, no statistically significant prognostic effect was found for molecular subtypes in patients >65 years of age (16). Therefore, it is worthwhile to conduct a study to evaluate the prognostic effect of molecular subtypes in young breast cancer patients (<40 years of age).
Multiple studies have indicated that young breast cancer patients are more likely to have a higher proportion of histological grade III (62-80%), negative ER and PR (33-80%), HER-2 overexpression (29-44%) (17,18), TNBC subtype (19,20), lymph node invasion and lymphovascular invasion (6,9,10,21,22). Our study was in agreement with the literature.
In our study, histological grade and tumor size (over 2 cm) were not associated with survival for young breast cancer patients. In the cases in our cohort, only 25 cases had a tumor mass over 5 cm and most cases were in the T1 and T2 stages with a mean tumor size of 2.83±1.53 cm. There was no significant difference in tumor size stage among the molecular subtypes in our study. Among 66 cases in the T1 stage, 23 were presented with positive lymph node status and 8 events of tumor relapse occurred. Shen indicated that in cases with 1-3 and 4-6 lymph nodes involved, the survival rate was not different when the tumor size was less than 5 cm, but there was a significant difference when the tumor size was larger than 5 cm (23). In addition, Wo et al. also found that very small tumors with 4 positive lymph nodes may predict for higher breast cancer specific mortality compared with larger tumors. In cases with extensive node-positive disease, very small tumor sizes may be a surrogate for biologically aggressive disease (24). Therefore, tumor size over 2 cm was not a prognostic factor in our study.
Our study also showed that BCT was not a risk factor for tumor relapse. Cao et al. examined 15-year outcomes among 616 women younger than 40 years old treated with BCT plus whole-breast radiation therapy, compared with 349 patients treated with modified radical mastectomy (MRM). The OS (74.2% vs. 73.0%, P=0.75), local relapse-free survival (85.4% vs. 86.5%, P=0.95) and distant relapse-free survival (74.4% vs. 71.6%, P=0.40) were similar between the BCT and MRM cohorts (25). There is another study showing a moderately higher local recurrence rate in young breast cancer patients compared with older patients after 5-10 year follow-up, but similar distant relapse-free survival rate and OS rate as well (26). Taken into account the psychological trauma of young patients as the result of mastectomy, we recommend BCT plus radiation therapy and long-term follow-up in appropriate cases.
In our study, 178 cases (95.2%) received chemotherapy. Therefore, chemotherapy status could not be included as a prognostic factor through statistical analysis because of the high rate of chemotherapy in our study. Agnese et al. supported chemotherapy in younger women even with node-negative tumors less than 1 cm in diameter (27). Clive et al. indicated that chemotherapy was increasingly being considered appropriate for all women under the age of 35 years old, regardless of other risk factors (28). DFS was obviously low in young patients without adjuvant chemotherapy (29). Kroman et al. found that young women with low risk disease who did not receive adjuvant chemotherapy had a significantly increased risk of dying. Risk increased with decreasing age at diagnosis [for patients <35 years: odds ratio (OR)=2.18, 95% confidence interval (95% CI): 1.64 to 2.89] even when women were grouped according to the presence of node negative disease and by tumor size (30). A meta-analysis by Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) indicated that the risk reductions through chemotherapy regimens were more apparent in young patients compared with older patients (31). In the 2013 St Gallen expert consensus, chemotherapy is needed in the TNBC subtype, HER-2 subtype, most cases of Luminal B subtype, and in cases of Luminal A subtype with high risk factors (such as grade III disease, involvement of 4 or more lymph nodes, etc.) (8). From the retrospective analysis, 9 cases who did not receive chemotherapy were in the Luminal subtype without high risk factors. Thus, all cases in our study underwent appropriate treatment in view of the current guidelines.
Other research groups have shown that the worse prognosis of young breast cancer patients was associated with a high proportion of ER negativity and HER-2 overexpression, which were strong prognostic factors (32,33). Furthermore, Ki67, which is closely related with cell proliferation, was also considered as an independent prognostic factor (34). The introduction of molecular subtypes in 2011, which integrates factors such as ER, PR, HER-2 and Ki67 as a whole, provided a more comprehensive prediction of breast cancer prognosis. Recent studies also indicated that lymph node metastasis, ER negativity and HER-2 overexpression were not all causes of worse prognosis in young patients. HER-2 and ER status were not independent prognostic factors. It was suggested that ER status was an important indicator for endocrine therapy rather than an independent prognostic factor and HER-2 overexpression was of no prognostic value in node negative cases (35).
Our study indicated that lymph node status and molecular subtypes were both prognostic factors of DFS and OS. In other studies, a statistically significant association (P=0.02) was found between molecular subtypes and age, where HER-2 and TNBC subtypes were more often found in young patients. Molecular subtypes showed a prognostic association with outcome in young patients with regards to relapse-free survival (P=0.01) and relative survival (P<0.001) (16). In the multivariate analysis, triple-negative status was the only independent prognostic factor which affected the DFS adversely [hazard ratio (HR): 1.48, 95% CI: 0.66-0.82, P=0.027] (36). In our study, relapse occurred in 8 cases in TNBC, among whom, 4 cases were node negative (50%). It was pointed out that TNBC had a worse prognosis without a very high rate of lymph node metastasis (37). Patients with TNBC are not more likely to have involved nodes than those with non-TNBC (38). Compared with HR(+)/HER-2(−) tumors as the reference group, TNBC subtype was associated with a lower risk of node positivity (OR=0.88, 95% CI: 0.80-0.97; P<0.001) (39). High risk of relapse still exists in young TNBC patients even with negative lymph nodes. It was reported that the 5-year OS in young TNBC patients with node negativity was as low as 80.5% (40). In patients with TNBC, once there was evidence of lymph node metastasis, the prognosis may not be affected by the number of positive lymph nodes (41). The accurate prediction of prognosis can be made using the integration of lymph node status and molecular subtype.
Our study showed the lowest DFS and OS in TNBC, followed by Luminal B subtype and HER-2 subtype. Among 29 cases which relapsed, 8 cases were in TNBC (27.6% of all relapses), which accounted for 25% of all cases in the TNBC subtype. Among the 13 cases that died, 5 cases were in TNBC (38.5% of all deaths), which accounted for 15.6% of all cases in the TNBC subtype. Eighteen cases in the HER-2 subtype (62.1%) received trastuzumab, which helped to improve their prognosis (42). While in the Luminal B subtype, the trastuzumab application rate was 26.3%, which maybe had a negative prognostic effect. High hormone levels and relatively insufficient ovarian suppression may contribute to a high relapse rate in young breast cancer patients (43). In standardized and personal treatments, more attention should be paid not only to the TNBC subtype, but also to the Luminal B subtype which accounts for the majority of young breast cancer patients (44).
There were also some limitations to our study, such as relatively small number of cases and selection bias, which was unavoidable because this was a retrospective cohort study. Most cases were in the early stages, of which TMN I and II stages accounted for the majority, which may attribute to the improvement of diagnosis and treatment, and more focus on breast disease. With more attention drawn towards the molecular subtypes in breast cancer, young patients will receive more appropriate personal treatment according to their predicted prognosis. Further investigation is warranted in the TNBC subtype which has a relatively worse prognosis, and in the Luminal B subtype as well, which currently lacks sufficient attention.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflict of interest to declare.
References
- Fan L, Strasser-Weippl K, Li JJ, et al. Breast cancer in China. Lancet Oncol 2014;15:e279-89. [PubMed]
- Tichy JR, Lim E, Anders CK. Breast cancer in adolescents and young adults: a review with a focus on biology. J Natl Compr Canc Netw 2013;11:1060-9. [PubMed]
- Ferlay J, Shin HR, Bray F, et al. GLOBOCAN 2008, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10. Lyon: International Agency for Research on Cancer; 2010. Available online: http://globocan.iarc.fr
- El Saghir NS, Seoud M, Khalil MK, et al. Effects of young age at presentation on survival in breast cancer. BMC Cancer 2006;6:194. [PubMed]
- Aebi S, Gelber S, Castiglione-Gertsch M, et al. Is chemotherapy alone adequate for young women with oestrogen-receptor-positive breast cancer? Lancet 2000;355:1869-74. [PubMed]
- Foo CS, Su D, Chong CK, et al. Breast cancer in young Asian women: study on survival. ANZ J Surg 2005;75:566-72. [PubMed]
- Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes--dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol 2011;22:1736-47. [PubMed]
- Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol 2013;24:2206-23. [PubMed]
- Han W, Kim SW, Park IA, et al. Young age: an independent risk factor for disease-free survival in women with operable breast cancer. BMC Cancer 2004;4:82. [PubMed]
- Rapiti E, Fioretta G, Verkooijen HM, et al. Survival of young and older breast cancer patients in Geneva from 1990 to 2001. Eur J Cancer 2005;41:1446-52. [PubMed]
- Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature 2000;406:747-52. [PubMed]
- Sørlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A 2001;98:10869-74. [PubMed]
- Keegan TH, DeRouen MC, Press DJ, et al. Occurrence of breast cancer subtypes in adolescent and young adult women. Breast Cancer Res 2012;14:R55. [PubMed]
- Morrison DH, Rahardja D, King E, et al. Tumour biomarker expression relative to age and molecular subtypes of invasive breast cancer. Br J Cancer 2012;107:382-7. [PubMed]
- Cancello G, Maisonneuve P, Rotmensz N, et al. Prognosis and adjuvant treatment effects in selected breast cancer subtypes of very young women (<35 years) with operable breast cancer. Ann Oncol 2010;21:1974-81. [PubMed]
- de Kruijf EM, Bastiaannet E, Rubertá F, et al. Comparison of frequencies and prognostic effect of molecular subtypes between young and elderly breast cancer patients. Mol Oncol 2014;8:1014-25. [PubMed]
- Lin CH, Lu YS, Huang CS, et al. Prognostic molecular markers in women aged 35 years or younger with breast cancer: is there a difference from the older patients? J Clin Pathol 2011;64:781-7. [PubMed]
- Anders CK, Hsu DS, Broadwater G, et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol 2008;26:3324-30. [PubMed]
- Carvalho FM, Bacchi LM, Santos PP, et al. Triple-negative breast carcinomas are a heterogeneous entity that differs between young and old patients. Clinics (Sao Paulo) 2010;65:1033-6. [PubMed]
- Anders CK, Fan C, Parker JS, et al. Breast carcinomas arising at a young age: unique biology or a surrogate for aggressive intrinsic subtypes? J Clin Oncol 2011;29:e18-20. [PubMed]
- Zhang M, Ning LS. The observation on lymph node metastasis and prognosis of the primary infiltrating breast cancer in women. Chinese Journal of Clinical Oncology 2006;33:91-2,95.
- Goksu SS, Tastekin D, Arslan D, et al. Clinicopathologic features and molecular subtypes of breast cancer in young women (age ≤35). Asian Pac J Cancer Prev 2014;15:6665-8. [PubMed]
- Shen ZZ. Relation of tumor size, lymph node status and prognosis in breast cancer. Zhonghua Wai Ke Za Zhi 1991;29:554-7. [PubMed]
- Wo JY, Chen K, Neville BA, et al. Effect of very small tumor size on cancer-specific mortality in node-positive breast cancer. J Clin Oncol 2011;29:2619-27. [PubMed]
- Cao JQ, Truong PT, Olivotto IA, et al. Should women younger than 40 Years of age with invasive breast Cancer have a mastectomy? 15-Year Outcomes in a population-based cohort. Int J Radiat Oncol Biol Phys 2014;90:509-17. [PubMed]
- Hwang ES, Lichtensztajn DY, Gomez SL, et al. Survival after lumpectomy and mastectomy for early stage invasive breast cancer: the effect of age and hormone receptor status. Cancer 2013;119:1402-11. [PubMed]
- Agnese DM, Yusuf F, Wilson JL. Trends in breast cancer presentation and care according to age in a single institution. Am J Surg 2004;188:437-9. [PubMed]
- Clive S, Dixon JM. The value of adjuvant treatment in young women with breast cancer. Drugs 2002;62:1-11. [PubMed]
- Xiong Q, Valero V, Kau V, et al. Female patients with breast carcinoma age 30 years and younger have a poor prognosis: the M.D. Anderson Cancer Center experience. Cancer 2001;92:2523-8. [PubMed]
- Kroman N, Jensen MB, Wohlfahrt J, et al. Factors influencing the effect of age on prognosis in breast cancer: population based study. BMJ 2000;320:474-8. [PubMed]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100000 women in 123 randomized trials. Lancet 2012;379:432-44. [PubMed]
- Liu W, Zhang XH, Zhang ZG, et al. The relationship between the Maspin, BCSG1 and c-erbB-2 expression and the prognosis of breast cancer. Chinese Journal of Clinical Oncology 2005;32:431-4.
- Maru D, Middleton LP, Wang S, et al. HER-2/neu and p53 overexpression as biomarkers of breast carcinoma in women age 30 years and younger. Cancer 2005;103:900-5. [PubMed]
- Colleoni M, Rotmensz N, Robertson C, et al. Very young women (<35 years) with operable breast cancer: features of disease at presentation. Ann Oncol 2002;13:273-9. [PubMed]
- Liu X, Liu QF, Xu Y, et al. Clinicopathologic characteristics and prognosis in young Chinese women with breast cancer. National Medical Journal of China 2011;91:1817-20. [PubMed]
- Koca B, Kuru B, Karabicak I, et al. Prognostic factors affecting disease-free survival in patients at age 35 or younger with invasive breast cancer. Ann Ital Chir 2014;85:249-53. [PubMed]
- Tischkowitz M, Brunet JS, Bégin LR, et al. Use of immunohistochemical markers can refine prognosis in triple negative breast cancer. BMC Cancer 2007;7:134. [PubMed]
- Gangi A, Mirocha J, Leong T, et al. Triple-negative breast cancer is not associated with increased likelihood of nodal metastases. Ann Surg Oncol 2014;21:4098-103. [PubMed]
- Lin NU, Vanderplas A, Hughes ME, et al. Clinicopathological features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Cancer 2012;118:5463-72. [PubMed]
- Zhang P, Xu BH, Ma F, et al. Clinicopathological characteristics and prognostic significance of young patients with triple-negative breast cancer. Zhonghua Zhong Liu Za Zhi 2010;32:128-31. [PubMed]
- Hernandez-Aya LF, Chavez-Macgregor M, Lei X, et al. Nodal status and clinical outcomes in a large cohort of patients with triple-negative breast cancer. J Clin Oncol 2011;29:2628-34. [PubMed]
- Su Y, Zheng Y, Zheng W, et al. Distinct distribution and prognostic significance of molecular subtypes of breast cancer in Chinese women: a population-based cohort study. BMC Cancer 2011;11:292. [PubMed]
- Shannon C, Smith IE. Breast cancer in adolescents and young women. Eur J Cancer 2003;39:2632-42. [PubMed]
- Sheridan W, Scott T, Caroline S, et al. Breast cancer in young women: have the prognostic implications of breast cancer subtypes changed over time? Breast Cancer Res Treat 2014;147:617-29. [PubMed]


