Prognostic value of clinicopathological characteristics in patients with pancreatic cancer
Introduction
The incidence of pancreatic cancer has gradually increased in recent years. Because of pancreatic cancer’s characteristics, including local invasion, being prone to distant metastasis, and drug resistance, this cancer has been considered a tumor with a poor prognosis over the past decade.
The median overall survival time after diagnosis is 6 months, and the 5-year survival rate is lower than 5% (1,2). Because this cancer is prone to local invasion and distant metastasis and is insensitive to drugs, surgery can only be performed in 20% of pancreatic ductal adenocarcinomas at the time of diagnosis. However, early recurrence or distant metastasis frequently occurs after surgery, and the 5-year survival rate of patients after surgery is rarely above 20%. Most patients miss the surgical opportunity because they already have local progression or distant metastasis at the time of diagnosis; therefore, the prognosis is poor (3). Currently, the treatment of ductal adenocarcinoma is based on the tumor stages, concomitant diseases, and performance status (PS) scores. Six months of gemcitabine-based chemotherapy after surgical resection is the standard for treating the early stages of pancreatic cancer (3). In contrast, patients with distant metastasis can only receive palliative chemotherapy. Although the treatment for patients with local progression but without distant metastasis remains debatable, single chemotherapy or radio-chemotherapy can be considered. There are various opinions regarding factors that affect overall survival time and prognosis. The purpose of this study was to analyze the effects of all clinical characteristics on the overall survival time, in order to provide a basis for determining the prognostic factor of patients with pancreatic cancer.
Patients and methods
A total of 103 pancreatic cancer patients were admitted to the Department of Radiotherapy and Chemotherapy of the Ruijin Hospital, Shanghai Jiaotong University School of Medicine, between January 2002 and December 2012. Inclusion criteria included histologically or cytologically proven pancreatic cancer, at least one measurable or evaluable lesion as assessed by CT-scan or MRI, and age ≥18 years. Among the assessed patients, those treatment with warfarin, uncontrolled hypercalcemia, known dihydropyrimidine dehydrogenase deficiency, other serious and uncontrolled non-malignant disease (e.g., active infection requiring systemic therapy, coronary stenting or myocardial infarction, or stroke in the past 6 months), HIV-infected patients or otherwise known to be HIV-positive with untreated hepatitis B or hepatitis C, medical history or active interstitial lung disease, other concomitant or previous malignancy (except adequately treated in-situ carcinoma of the uterine cervix, basal or squamous cell carcinoma of the skin, cancer in complete remission for >5 years) were excluded. There were 68 men and 35 women; the median age was 62 years. The clinical symptoms included 13 cases (12.62%) of nausea and vomiting, 87 cases (84.47%) of abdominal pain and abdominal distension, 43 cases (41.75%) of lower back pain, six cases (5.83%) of diarrhea, 83 cases (80.58%) of loss of appetite, 39 cases (37.86%) of body weight loss >10%, and 54 cases (52.43%) of body weight loss <10%. During disease onset, 32 patients (31.07%) presented with diabetes mellitus, 10 (9.71%) had ascites, 25 (24.27%) had jaundice, 50 (48.54%) had liver metastasis, 11 (10.68%) had supraclavicular lymph node metastasis, 43 (41.75%) had carcinoma of the head and neck of the pancreas, and 60 (58.25%) had carcinoma of the body and tail of the pancreas. For the American Joint Committee on Cancer (AJCC) stages, there are 1 (0.97%) stage II cases, 35 (33.98%) stage III cases, and 67 cases (65.05%) stage IV cases.
In the case of elevated preoperative serum CA19.9 levels the assessment of this marker was performed every 3 months for 2 years and an abdominal CT scan every 6 months. The median overall survival was defined as the interval between the date of beginning of treatment to death or last follow-up visit, and to clinical progression or death or last follow-up visit if not progressed. The survival time was calculated from the date of confirmed pathological diagnosis to the time of death, or the final follow-up date. The study was conducted with the approval of the institutional ethics board of our institute.
Statistical analysis
SPSS13.0 statistical software was used. The Kaplan-Meier method was performed to calculate the overall survival rate. The log-rank method was used to examine the univariate analysis. The Cox regression model was performed for multivariate analysis. P<0.05 was considered statistically significant.
Results
Laboratory tests
During the disease onset in the 103 patients, 19 (18.45%) had increased total white blood cell (WBC) count, 8 (7.77%) had decreased WBC count, 57 (55.34%) had decreased hemoglobin, 11 (10.68%) had an increased platelet count, and 10 (9.71%) had a decreased platelet count. Alanine aminotransferase levels were increased in 100 cases (10.68%), serum total bilirubin levels were increased in 35 cases (33.98%), creatinine levels were increased in 7 cases (6.80%), creatinine levels were decreased in 17 cases (16.50%), albumin levels were decreased in 43 cases (41.75%), and carcinoembryonic antigen (CEA) levels were increased in 54 cases (52.43%). Sixty-nine cases (66.99%) had >300 U/L carbohydrate antigen 19-9 (CA199); 20 cases (19.42%) had increased CA199, although the level was <300 U/L.
Treatment methods
Among these patients, 21 (20.39%) received radical surgery for pancreatic cancer, 13 (12.62%) received palliative surgery, and 69 (66.99%) did not receive surgery. Eighty-three patients (80.58%) received chemotherapy; 68 of those patients (66.02%) received chemotherapy alone, 15 (14.56%) received chemotherapy combining with local radiotherapy, two (1.94%) received radiotherapy alone, and 18 (17.48%) received the best supportive care. The first-line treatment of 63 patients (61.17%) was gemcitabine; 20 patients (19.42%) were administered other drugs for the first-line treatment [including 5-fluorouracil (5-FU), Xeloda, TS-1, and irinotecan].
Overall survival and prognostic factors
The median survival time was 293 days, the 1-, 2-, and 3-year survival rates were 27.18%, 5.83%, and 1.94%, respectively. The patients with good physical strength had significantly longer survival times than those with poor physical strength (P<0.05). Patients older than 55 years with left supraclavicular lymph node enlargement, accompanying ascites, or liver metastasis had shorter survival times. In addition, patients with a body weight loss >10% also had shorter survival times. The survival time of one stage II patient reached 1,290 days, the median survival time of 35 phase III patients was 718 days, and the median survival time of 67 stage IV patients was 189 days (Table 1). The survival time of stage III patients was longer than the survival time of stage IV patients (P<0.001).
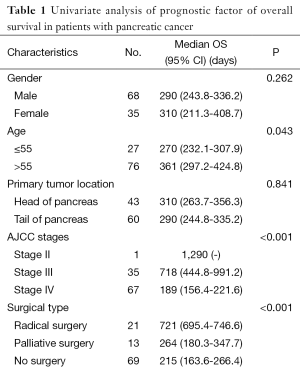
Full table
The gender, tumor location, accompanying diabetes mellitus, symptoms such as nausea and vomiting, abdominal pain and abdominal distension, lower back pain, diarrhea, and yellow discoloration of skin during physical examination did not affect the overall survival (P>0.05). The median survival time of patients with normal tumor indicator CEA was 377 days, and the median survival time of patients with increased CEA was 201 days; there was a significant difference between these two (P=0.001). The median survival time of patients with CA199 >300 U/L, increased CA199 but <300 U/L, and normal CA199 was 215 days, 524 days, and 718 days, respectively; there was a significant difference between the former and the latter two (P=0.001 and P=0.003). The survival times of patients with increased CA199 but <300 U/L and normal CA199 did not indicate a significant difference (P=0.569).
Patients with increased serum total bilirubin, decreased albumin, and increased creatinine and patients with increased WBC count and abnormal platelets had shorter survival times (P<0.05). Changes in the biochemical indicator alanine aminotransferase did not significantly influence survival time (P>0.05). The median survival time of patients who received radical surgery was 721 days, the median survival time of patients who received palliative treatment was 264 days, and the median survival time of patients without surgery was 215 days. The survival time of patients who received radical surgery was thus significantly lengthened compared with patients receiving palliative treatment and patients who did not undergo surgery (P=0.000 and P=0.000); there was no difference in the survival time of patients who received palliative treatment and patients who did not undergo surgery (P=0.721).
The survival time of patients who received chemotherapy or radio-chemotherapy was longer than the survival time of patients who did not receive radio-chemotherapy (P=0.005 and P=0.004); the survival time of patients who received chemotherapy alone was not different from patients who received radio-chemotherapy (P=0.985). Among patients who received chemotherapy, the survival times of patients who received the gemcitabine treatment program as the first-line treatment (326 days) and patients who received other first-line treatment (252 days) were not statistically different (P=0.221).
The 16 factors that were significant were introduced into the Cox regression equation, the results indicate that age (P=0.015), Karnofsky PS (P=0.002), surgical types (P<0.001), and platelet counts (P<0.001) were independent prognostic factors affecting the overall survival of pancreatic cancer patients (Table 2).
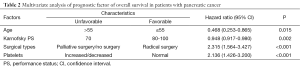
Full table
Discussion
The Pancreatic Cancer Committee of the Chinese Anti-Cancer Association conducted a clinical epidemiological survey on pancreatic cancer. A total of 2,340 patients with pancreatic cancer from 13 large centers in China were analyzed. The median survival times of stages I, II, III, and IV patients were 22.80, 19.57, 8.45, and 5.44 months, respectively. The 1-year survival rates of these groups were 64.58%, 60.82%, 17.29%, and 8.79%, respectively; the 3-year survival rates were 26.04%, 22.68%, 0%, and 0%, respectively; the 5-year survival rates were 15.63%, 10.31%, 0%, and 0%, respectively. The median overall survival time of patients with carcinoma of the head of the pancreas, and carcinoma of the body and tail of the pancreas was 9.88 and 8 months, respectively. The median survival times of patients who received radical surgery and palliative treatment were 20.14 and 7.36 months, respectively (4). The survival analysis of 204 cases of pancreatic cancer reported by Zhang et al. showed that the median survival time was 6.4 months and that the 1-, 2-, and 3-year survival rates were 32.8%, 13.7%, and 2.9%, respectively (5). Among the 103 cases of pancreatic cancer in this study, the median overall survival time was 293 days, and the 1-, 2-, and 3-year overall survival rates were 27.18%, 5.83%, and 1.94%, respectively. The overall survival rates in this study were lower than the survival rates in the above reports (4,5). The reason may be there were more stage IV patients (65.05%) and patients who did not undergo radical surgery for pancreatic cancer (79.61%) in this study.
This study showed that clinical symptoms are accompanied by enlargement of the left supraclavicular lymph node, ascites, liver metastasis, late staging, poor KPS, older age, not being suitable for radical surgery, and short overall survival of patients without radiotherapy and chemotherapy. In addition, the results showed that patients with increased CA199, increased CEA, increased WBC, increased hemoglobin, decreased platelets, increased total bilirubin, decreased albumin, and increased creatinine had shorter survival times. The common clinical symptoms in general pancreatic cancer such as nausea, vomiting, abdominal pain and abdominal distension, lower back pain, and diarrhea did not affect the prognosis.
It was reported that the mean survival time of carcinoma of the head of the pancreas was longer than the survival time of carcinoma of the body and tail of the pancreas (6-8). The survival times of patients with carcinoma of the head of the pancreas and carcinoma of the body and tail of the pancreas in this study were not significantly different. Poruk et al. retrospectively analyzed the pancreatic cancer-related tumor indicators and showed that the sensitivity and specificity of CA199 were 78.2% and 82.8%, respectively, and that the sensitivity and specificity of CEA were 44.2% and 84.8%, respectively (9). Boeck et al. showed that for patients in the advanced stage, an increase in CA199 levels influenced survival time; in addition, a decrease in CA199 levels within 8 weeks following chemotherapy may predict whether the treatment was effective (10). In this study, we found that when the tumor indicators CEA and CA199 in patients increased, the mean survival time significantly decreased. In particular, when CA199 >300 U/L, the prognosis was poor, and the mean survival time was only 215 days.
Studies have shown that patients with increased WBC and decreased platelets have a shorter survival time (11,12). Increased WBC or increased platelets in many patients with advanced tumors may be associated with the secretion of factors such as G-CSF and GM-CSF by tumors (11-13). In this study, it was found that an abnormal platelet count was an independent prognostic factor affecting the survival of pancreatic cancer patients. Generally, patients with low platelet counts cannot tolerate surgery, chemotherapy, or radiotherapy; such patients can only receive symptomatic therapy. The survival time of patients at the advanced stage is also shorter than for patients who receive radiotherapy and chemotherapy.
Currently, surgery remains the most effective treatment for pancreatic cancer, the group of patients having radical surgery for pancreatic cancer had significantly improved survival times, compared with the palliative treatment group and the non-surgery group (14,15). This study showed that the median survival time of patients who received radical surgery was 721 days, whereas the patients who received palliative treatment and those who did not undergo surgery was 264 and 215 days, respectively. The survival time of the former was significantly longer, which was consistent with the previous studies (14,15). The effects of chemotherapy, combined radiotherapy and chemotherapy treatment, and a variety of chemotherapy programs on the survival times of pancreatic cancer patients are not clear. Many clinical trials have used gemcitabine to combine with other chemotherapeutic drugs, including 5-FU (16), capecitabine (17), oxaliplatin (18,19), cisplatin (20,21), irinotecan (22), and pemetrexed (23), to treat advanced pancreatic cancer. Although some trial results have indicated small improvements in progression-free survival, there was no improvement in overall survival (24,25). The PRODIGE clinical trial used the FOLFIRINOX three-drug program to treat advanced pancreatic cancer using gemcitabine as a control. The overall survival rate in the three-drug program reached 11.1 months, whereas the overall survival rate using gemcitabine alone was 6.8 months (26). This was the first study using a non-gemcitabine chemotherapy program to achieve an increase in the survival time of patients with advanced pancreatic cancer. However, only patients with good physical strength can tolerate the treatment of three drugs and benefit from such a protocol. Only in a phase III combined chemotherapy clinical trial (27) did the small molecule inhibitor of the EGFR receptor pathway erlotinib prolong the median survival time for two weeks compared with patients in the placebo group. In this study, the median survival rate of patients who received gemcitabine as the first-line treatment was 326 days, whereas the median survival rate of patients using other drugs (e.g., 5-FU, capecitabine, oxaliplatin, irinotecan, or TS-1) as the first-line treatment was 252 days.
In conclusion, pancreatic cancer had a poor prognosis, the general physical condition, age, the availability of radical surgery, and platelet counts were factors influencing the overall survival of patients with pancreatic cancer.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [PubMed]
- Zaccagna F, Anzidei M, Sandolo F, et al. MRgFUS for liver and pancreas cancer treatments: the Umberto I hospital experience. Transl Cancer Res 2014;3:430-41.
- Bao B, Azmi AS, Ali S, et al. Metformin may function as anti-cancer agent via targeting cancer stem cells: the potential biological significance of tumor-associated miRNAs in breast and pancreatic cancers. Ann Transl Med 2014;2:59. [PubMed]
- Chen W, Zheng R, Zhang S, et al. Annual report on status of cancer in China, 2010. Chin J Cancer Res 2014;26:48-58. [PubMed]
- Zhang DX, Yuan SX, Dai YD. Survival analysis of 204 patients with pancreatic cancer. Chinese Journal of Digestion 2010;30:236-40. (in Chinese).
- Wu HX, Dai YD, Zhang DX, et al. The relation of lactic dehydrogenase and tumor markers with pancreatic cancer. Journal of Modern Oncology 2012;20:1392-7. (in Chinese).
- Dai YD, Zhang DX, Yuan SX, et al. Treatment and prognostic factors affecting on pancreatic cancer survival. China Oncology 2011;21:211-5. (in Chinese).
- Hua YP, Liang LJ, Peng BG, et al. Factors influencing the prognosis of 276 patients with pancreatic cancer. Chinese Journal of Digestive Surgery 2008;7:413-5. (in Chinese).
- Poruk KE, Gay DZ, Brown K, et al. The clinical utility of CA 19-9 in pancreatic adenocarcinoma: diagnostic and prognostic updates. Curr Mol Med 2013;13:340-51. [PubMed]
- Boeck S, Stieber P, Holdenrieder S, et al. Prognostic and therapeutic significance of carbohydrate antigen 19-9 as tumor marker in patients with pancreatic cancer. Oncology 2006;70:255-64. [PubMed]
- Aliustaoglu M, Bilici A, Seker M, et al. The association of pre-treatment peripheral blood markers with survival in patients with pancreatic cancer. Hepatogastroenterology 2010;57:640-5. [PubMed]
- Wang DS, Luo HY, Qiu MZ, et al. Comparison of the prognostic values of various inflammation based factors in patients with pancreatic cancer. Med Oncol 2012;29:3092-100. [PubMed]
- Lee KC, Maturo C, Perera CN, et al. Translational assessment of mitochondrial dysfunction of pancreatic cancer from in vitro gene microarray and animal efficacy studies, to early clinical studies, via the novel tumor-specific anti-mitochondrial agent, CPI-613. Ann Transl Med 2014;2:91. [PubMed]
- Lloyd S, Chang BW. A comparison of three treatment strategies for locally advanced and borderline resectable pancreatic cancer. J Gastrointest Oncol 2013;4:123-30. [PubMed]
- Tol JA, Eshuis WJ, Besselink MG, et al. Non-radical resection versus bypass procedure for pancreatic cancer - a consecutive series and systematic review. Eur J Surg Oncol 2015;41:220-7. [PubMed]
- Berlin JD, Catalano P, Thomas JP, et al. Phase III study of gemcitabine in combination with fluorouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern Cooperative Oncology Group Trial E2297. J Clin Oncol 2002;20:3270-5. [PubMed]
- Herrmann R, Bodoky G, Ruhstaller T, et al. Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: a randomized, multicenter, phase III trial of the Swiss Group for Clinical Cancer Research and the Central European Cooperative Oncology Group. J Clin Oncol 2007;25:2212-7. [PubMed]
- Louvet C, Labianca R, Hammel P, et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol 2005;23:3509-16. [PubMed]
- Poplin E, Feng Y, Berlin J, et al. Phase III, randomized study of gemcitabine and oxaliplatin versus gemcitabine (fixed-dose rate infusion) compared with gemcitabine (30-minute infusion) in patients with pancreatic carcinoma E6201: a trial of the Eastern Cooperative Oncology Group. J Clin Oncol 2009;27:3778-85. [PubMed]
- Heinemann V, Quietzsch D, Gieseler F, et al. Randomized phase III trial of gemcitabine plus cisplatin compared with gemcitabine alone in advanced pancreatic cancer. J Clin Oncol 2006;24:3946-52. [PubMed]
- Colucci G, Giuliani F, Gebbia V, et al. Gemcitabine alone or with cisplatin for the treatment of patients with locally advanced and/or metastatic pancreatic carcinoma: a prospective, randomized phase III study of the Gruppo Oncologia dell'Italia Meridionale. Cancer 2002;94:902-10. [PubMed]
- Rocha Lima CM, Green MR, Rotche R, et al. Irinotecan plus gemcitabine results in no survival advantage compared with gemcitabine monotherapy in patients with locally advanced or metastatic pancreatic cancer despite increased tumor response rate. J Clin Oncol 2004;22:3776-83. [PubMed]
- Oettle H, Richards D, Ramanathan RK, et al. A phase III trial of pemetrexed plus gemcitabine versus gemcitabine in patients with unresectable or metastatic pancreatic cancer. Ann Oncol 2005;16:1639-45. [PubMed]
- Heinemann V, Labianca R, Hinke A, et al. Increased survival using platinum analog combined with gemcitabine as compared to single-agent gemcitabine in advanced pancreatic cancer: pooled analysis of two randomized trials, the GERCOR/GISCAD intergroup study and a German multicenter study. Ann Oncol 2007;18:1652-9. [PubMed]
- Sultana A, Smith CT, Cunningham D, et al. Meta-analyses of chemotherapy for locally advanced and metastatic pancreatic cancer. J Clin Oncol 2007;25:2607-15. [PubMed]
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25. [PubMed]
- Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2007;25:1960-6. [PubMed]


