Addition of rituximab is not associated with survival benefit compared with CHOP alone for patients with stage I diffuse large B-cell lymphoma
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common non-Hodgkin lymphoma (1,2). Several randomized phase III clinical trials have demonstrated that the addition of rituximab to the traditional cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP) regimen could significantly improve survival of DLBCL patients (3-7).
However, patients with stage I disease were not fully analyzed in any of these phase III clinical trials and whether rituximab contained chemotherapy could improve survival of these patients remains unclear. MabThera International Trial (MInT) was a phase III clinical trial demonstrated that CHOP plus rituximab (R-CHOP) was associated with better survival compared with CHOP alone in young DLBCL patients with good-prognosis. A total of 824 patients with stage II-IV, or stage I with bulky disease were enrolled. But the number of stage I patients was limited and the stage I patients without bulky disease were not enrolled (5). The prospective phase II study showed that the addition of rituximab could improve survival, but this result was only compared with historical study (8). So the role of rituximab for patients with stage I DLBCL in previous studies was not persuasive. Therefore, paralleled controlled study was needed to assess whether CHOP plus rituximab (R-CHOP) can improve outcome compared with CHOP alone for stage I DLBCL patients.
A randomized phase III study compared three cycles of CHOP followed by involved-field radiotherapy (IFRT) with eight cycles of CHOP alone which demonstrated that three cycles of CHOP followed by IFRT were associated with survival benefit. Three cycles of CHOP followed by IFRT had been the standard therapy for patients with limited stage DLBCL in the pre-rituximab era (9). In the rituximab era, phase II clinical trial demonstrated that R-CHOP plus IFRT was associated with better survival outcome compared with historical result of CHOP plus IFRT (8). The results presented in a retrospective study suggested that using six cycles of full-dose R-CHOP treatment alone without radiotherapy could be effective in patients with limited stage (10). Therefore, whether the addition of IFRT could improve survival of stage I patients especially those received R-CHOP chemotherapy remains to be defined. This study also evaluated the role of IFRT in these patients.
Materials and methods
Patients
Between 2003 and 2009, 140 untreated patients with stage I DLBCL treated in Cancer Hospital, Academy of Medical Sciences (CAMS) & Peking Union Medical College (PUMC) were included in this study. The eligibility criteria for patients in this study included: (I) histological diagnosis of DLBCL based on the WHO classification; (II) stage I according to the Ann Arbor system (11); (III) no previous therapy; and (IV) received CHOP or R-CHOP in first-line treatment. We excluded patients from the study if they had: (I) severe infection; (II) congestive heart failure; (III) active hepatitis B virus infection; (IV) deficient hematologic, hepatic, and renal functions; (V) previous treatment with chemotherapy or radiotherapy; or (VI) received chemotherapy other than CHOP or R-CHOP in first-line treatment. All pathological specimens were reviewed in order to confirm the diagnosis in our institution. The immunohistochemical stains for CD10, BCL-6 and MUM1 were evaluated to determine whether the patients belong to germinal center or non-germinal center subgroup. A bulky mass was defined as any mass exceeding 10 cm in diameter in a horizontal plane or a mediastinal mass with a maximum diameter exceeding one-third of the maximum chest diameter as observed on CT scans. The study was approved by the Ethics Committee of the Cancer Hospital, CAMS & PUMC, and written informed consent of all eligible patients was obtained.
Chemotherapy
All the patients were scheduled to undergo primary therapy with R-CHOP [75 mg/m2 epirubicin, 750 mg/m2 cyclophosphamide, 1.4 mg/m2 (maximum 2.0 mg/body) vincristine, 500 mg/body of prednisolone, and 375 mg/m2 rituximab every 21 d per cycle] or CHOP regimen [75 mg/m2 epirubicin, 750 mg/m2 cyclophosphamide, 1.4 mg/m2 (maximum 2.0 mg/body) vincristine, and 500 mg/body of prednisolone every 21 days per cycle].
Response and follow-up criteria
According to International Workshop Criteria, initial response includes complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD). Overall survival (OS) was calculated from the date of initiation of chemotherapy to the date of either the last follow-up or death. Patients who were alive at the last follow-up visit were referred to as censored data. Progression-free survival (PFS) was calculated from the date of initiation of chemotherapy to the date of disease progression, deaths or last follow-up. Patients who were not progressed or died at the last follow-up were recognized as censored data.
Statistical analysis
Comparisons of clinical characteristics and CR rate between R-CHOP and CHOP groups were performed using χ2 test and Wilcoxon Rank-Sum test. Survival was estimated by the Kaplan-Meier method. Log-rank test was used for univariate analysis, and P<0.05 (two-sided test) was considered statistically significant. The Cox proportional hazards model was used for multivariate analysis. P<0.05 (two-sided test) was considered to indicate statistical significance. Statistical analysis was conducted using the SPSS 19.0 software package (SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics
A total of 140 patients were enrolled in our study with 78 patients in CHOP group and 62 patients in R-CHOP group. The R-CHOP and CHOP groups were well-balanced with respect to baseline characteristics. Forty-two (68%) and 49 (63%) patients received radiotherapy in R-CHOP group and CHOP group respectively (P=0.554). Eight (13%) and 7 (9%) patients had bulky mass in R-CHOP and CHOP groups respectively (P=0.455). The median number of chemotherapy cycles in both R-CHOP and CHOP groups was 4 (P=0.096) (Table 1). Patients who received combined chemoradiotherapy and chemotherapy alone were also well-balanced with respect to baseline characteristics. Forty-two patients (46%) in radiotherapy group and 20 patients (41%) in no radiotherapy group received R-CHOP chemotherapy (P=0.544).
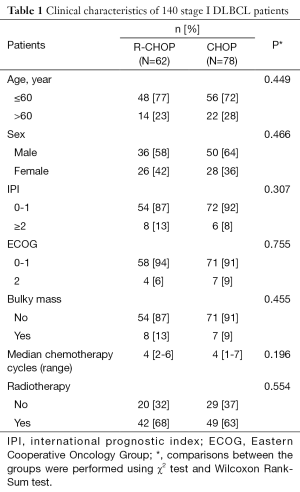
Full table
Response
At the end of initial treatment, the CR rate was 77% both in R-CHOP and CHOP groups (P=0.945). The PR rate was 15% in two groups. The SD rate was 3% in R-CHOP group and 4% in CHOP group. PD occurred in 5% of patients treated with R-CHOP and 4% of patients with CHOP regimen (Table 2).
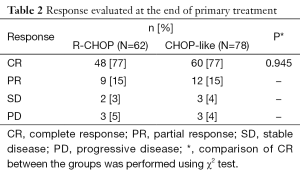
Full table
Follow-up and survival
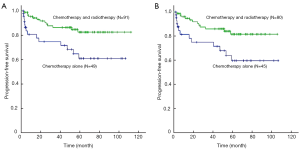
After a median follow-up period of 56 months, patients received R-CHOP had similar 5-year PFS (76% vs. 85%; log-rank P=0.215) and 5-year OS (90% vs. 96%; log-rank P=0.175) compared with those with CHOP alone (Figure 1). Concerning the 125 patients with non-bulky stage I DLBCL alone, there was also no significant difference of 5-year PFS (76% vs. 83%; log-rank P=0.301) and OS (91% vs. 96%; log-rank P=0.247) rate in patients treated with R-CHOP and CHOP regimen alone (Figure 2). For 91 patients who received radiotherapy, there was also no difference of 5-year OS (95% vs. 98%; log-rank P=0.503) and 5-year PFS (86% vs. 86%; log-rank P=0.901) in R-CHOP and CHOP groups. Subgroup analysis showed that both in favorable subgroup [i.e., international prognostic index (IPI) =0, no bulk] and less-favorable subgroup (i.e., IPI≥1, or bulk, or both), patients received R-CHOP regimen were not associated with superior 5-year PFS rate compared with those with CHOP alone. All stage I (86% vs. 71%; log-rank P=0.005) and non-bulky stage I (85% vs. 71%; log-rank P=0.009) DLBCL patients who received radiotherapy had significantly increased 5-year PFS compared with those who received chemotherapy alone (Figure 3). For 62 patients received R-CHOP chemotherapy, radiotherapy could also significantly improve 5-year PFS (86% vs. 55%; log-rank P<0.001) and 5-year OS (95% vs. 80%; log-rank P=0.016).
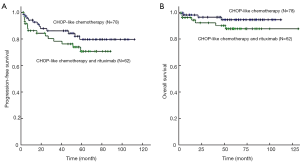
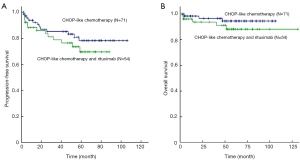
Prognostic factors
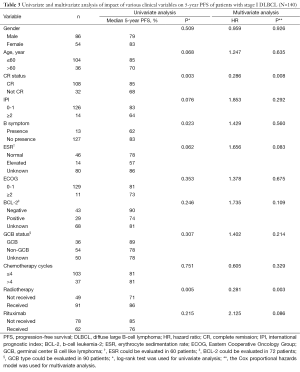
Within the whole cohort, the factors significantly associated with a superior PFS were patients who had CR after initial therapy (P=0.003), did not have B symptoms (P=0.023) and received radiotherapy (P=0.005) at univariate analysis. At multivariate analysis, patients who had CR (P=0.008) and received radiotherapy (P=0.003) were significantly associated with superior PFS (Table 3).
Full table
Discussion
Our study evaluated the role of rituximab and IFRT for stage I or stage I non-bulky patients. In our study, the addition of rituximab was not associated with survival benefit compared with CHOP alone and additional IFRT could significantly improve survival for DLBCL patients with stage I or stage I non-bulky disease.
Several randomized phase III clinical trials have evaluated whether the addition of rituximab to the traditional CHOP regimen could improve survival of DLBCL patients (3-7). Two randomized controlled, multicenter phase III clinical trials, LNH-98.5 and RICOVER-60 studies, only confirmed the benefit of rituximab for elderly patients (4,6). MInT compared CHOP and R-CHOP regimens in young DLBCL patients with good prognosis. A total of 824 patients who had no risk factors or one risk factor according to age-adjusted IPI (aaIPI), stage II-IV disease, or stage I disease with bulk were enrolled (5). But the number of stage I patients was limited and patients with stage I and non-bulky disease were not included in MInT study or any other phase III clinical trials. The prospective phase II study showed that the addition of rituximab could improve survival, but this result was only compared with historical study (8). So whether rituximab-contained chemotherapy can improve survival of patients with stage I or stage I non-bulky DLBCL remains to be assessed in paralleled controlled study.
In this study, patients received R-CHOP regimen had similar 5-year PFS and OS compared with those with CHOP alone. These results suggested that CHOP alone might be as effective as R-CHOP for patients with stage I or stage I non-bulky DLBCL patients. The survival benefit of rituximab for DLBCL patients was not as significant as reported in previous studies might be for the reason that all cases included in our study were stage I DLBCL patients for whom chemotherapy alone could have favorable outcome.
MInT study showed that a favorable subgroup (i.e., IPI=0, no bulk) could be defined from a less-favorable subgroup (i.e., IPI=1 or bulk, or both) (3). The patients in our study were also divided into favorable subgroup (i.e., IPI=0, no bulk) and less-favorable subgroup (i.e., IPI=1 or bulk, or both) in the subgroup analysis. It could be found that in both subgroups the addition of rituximab could not be associated with survival benefits. Other prognostic factors such as BCL-2, subtype of pathology, B symptoms and erythrocyte sedimentation rate (ESR) had been reported in previous studies (12,13). But owing to the limited sample, we did not incorporate these factors into subgroup analysis.
It has been demonstrated that three cycles of CHOP followed by IFRT was associated with survival benefit (9). In the prospective phase II SWOG 0014 study, three courses of R-CHOP followed by IFRT in high-risk patients was compared with the results of SWOG 8736 study, and the addition of rituximab could improve survival (8). The results presented in a retrospective study suggested that using six cycles of full-dose R-CHOP treatment alone without radiotherapy could be beneficial and effective in patients with limited stage DLBCL (10). But other studies demonstrated that patients with limited stage could benefit from radiotherapy (14). So whether IFRT after chemotherapy could improve survival compared with chemotherapy alone in stage I or stage I non-bulky DLBCL patients especially patients received R-CHOP regimen remains to be assessed. In this study, 91 patients received additional radiotherapy at the end of chemotherapy. Patients with radiotherapy had significantly increased 5-year PFS compared with those who had chemotherapy alone. Concerning the patients with non-bulky stage I DLBCL alone, patients received radiotherapy also significantly increased 5-year PFS. To reduce the interference of rituximab, we analyzed 62 patients received R-CHOP chemotherapy, and radiotherapy could also significantly improve 5-year PFS and 5-year OS. These results suggested that additional radiotherapy is still necessary for stage I or stage I non-bulky DLBCL patients even for patients received standard R-CHOP chemotherapy.
In this study, the factors significantly associated with a superior 5-year PFS were patients who had CR after initial therapy, and received radiotherapy at multivariate analysis. The results above were corresponded with previous studies (3,5,12,15-17). This study also found that patients with more than 4 cycles of chemotherapy were not associated with survival benefit which suggested that 4 cycles of chemotherapy might be enough for stage I DLBCL patients. The results of multivariate analysis in our study further confirmed that R-CHOP chemotherapy might not improve outcome compared with CHOP alone and radiotherapy could significant improve PFS for stage I DLBCL patients.
This is a retrospective study so the bias between R-CHOP and CHOP groups exists. Besides, whether the patients received radiotherapy or not could be a confounding factor when comparing the role of rituximab. To reduce the imbalance, the interclass equilibration were compared which showed that the different treatment groups were well-balanced with respect to baseline characteristics. Concerning 91 patients who received radiotherapy, there was also no difference in 5-year OS and 5-year PFS between R-CHOP and CHOP groups. Multivariate analysis was also performed to reduce the bias. Now the studies assessing the role of rituxiamab for stage I DLBCL patients are limited and a long period would be needed to wait for the results of prospective studies. The sample size in this study is not large enough, but with regard to the results in our study, new problems have been raised which needs further evaluation in prospective, randomized, phase 3 clinical trials.
Conclusions
CHOP alone could be as effective as R-CHOP and additional IFRT would be necessary for stage I or stage I non-bulky DLBCL patients. Patients who had CR and received radiotherapy were significantly associated with longer PFS at multivariate analysis. Even though the survival benefit of rituximab for stage I DLBCL patients was not as significant as expected in our study, we still could not deny the importance of rituximab for DLBCL patients of limited stage. New problems have been raised with regard to the results in our study which need further evaluation in prospective, randomized, phase 3 clinical trials.
Acknowledgements
The authors acknowledge the contributions of Departments of Medical Oncology and Radiation Oncology for study collaboration, and Department of Medical Record Library for medical record provision, and thank Chinese Society of Clinical Oncology (CSCO) for partial financial support.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Armitage JO, Weisenburger DD. New approach to classifying non-Hodgkin's lymphomas: clinical features of the major histologic subtypes. Non-Hodgkin's Lymphoma Classification Project. J Clin Oncol 1998;16:2780-95. [PubMed]
- Chen W, Zheng R, Zeng H, et al. Annual report on status of cancer in China, 2011. Chin J Cancer Res 2015;27:2-12. [PubMed]
- Pfreundschuh M, Kuhnt E, Trümper L, et al. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol 2011;12:1013-22. [PubMed]
- Pfreundschuh M, Schubert J, Ziepert M, et al. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60). Lancet Oncol 2008;9:105-16. [PubMed]
- Pfreundschuh M, Trümper L, Osterborg A, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol 2006;7:379-91. [PubMed]
- Coiffier B, Thieblemont C, Van Den Neste E, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d'Etudes des Lymphomes de l'Adulte. Blood 2010;116:2040-5. [PubMed]
- Coiffier B. State-of-the-art therapeutics: diffuse large B-cell lymphoma. J Clin Oncol 2005;23:6387-93. [PubMed]
- Persky DO, Unger JM, Spier CM, et al. Phase II study of rituximab plus three cycles of CHOP and involved-field radiotherapy for patients with limited-stage aggressive B-cell lymphoma: Southwest Oncology Group study 0014. J Clin Oncol 2008;26:2258-63. [PubMed]
- Miller TP, Dahlberg S, Cassady JR, et al. Chemotherapy alone compared with chemotherapy plus radiotherapy for localized intermediate- and high-grade non-Hodgkin's lymphoma. N Engl J Med 1998;339:21-6. [PubMed]
- Tomita N, Takasaki H, Miyashita K, et al. R-CHOP therapy alone in limited stage diffuse large B-cell lymphoma. Br J Haematol 2013;161:383-8. [PubMed]
- Smithers DW. Summary of papers delivered at the Conference on Staging in Hodgkin’s Disease (Ann Arbor). Cancer Res 1971;31:1869-70. [PubMed]
- Iqbal J, Meyer PN, Smith LM, et al. BCL2 predicts survival in germinal center B-cell-like diffuse large B-cell lymphoma treated with CHOP-like therapy and rituximab. Clin Cancer Res 2011;17:7785-95. [PubMed]
- Pfreundschuh M, Ho AD, Cavallin-Stahl E, et al. Prognostic significance of maximum tumour (bulk) diameter in young patients with good-prognosis diffuse large-B-cell lymphoma treated with CHOP-like chemotherapy with or without rituximab: an exploratory analysis of the MabThera International Trial Group (MInT) study. Lancet Oncol 2008;9:435-44. [PubMed]
- Ballonoff A, Rusthoven KE, Schwer A, et al. Outcomes and effect of radiotherapy in patients with stage I or II diffuse large B-cell lymphoma: a surveillance, epidemiology, and end results analysis. Int J Radiat Oncol Biol Phys 2008;72:1465-71. [PubMed]
- Phan J, Mazloom A, Medeiros LJ, et al. Benefit of consolidative radiation therapy in patients with diffuse large B-cell lymphoma treated with R-CHOP chemotherapy. J Clin Oncol 2010;28:4170-6. [PubMed]
- Ying Z, Wang X, Song Y, et al. Prognostic value of interim (18)F-FDG PET/CT in diffuse large B-cell lymphoma. Chin J Cancer Res 2013;25:95-101. [PubMed]
- Jia B, Shi Y, Dong M, et al. Clinical features, survival and prognostic factors of primary testicular diffuse large B-cell lymphoma. Chin J Cancer Res 2014;26:459-65. [PubMed]


