Breast-conserving therapy and modified radical mastectomy for primary breast carcinoma: a matched comparative study
Introduction
In 1990, the National Institutes of Health (NIH) released a consensus statement recommending the use of breast-conserving surgery (BCS) with adjuvant radiotherapy instead of mastectomy for the treatment of early-stage (stage I or II) breast cancer, whenever possible (1). For women diagnosed with early-stage breast cancer, survival with breast-conserving therapy (BCT) is comparable to that achieved with mastectomy following initial treatment (2), and BCT may afford better body image and sexual function (3). BCT is becoming a widely used therapy for breast cancer, as seven prospectively randomized studies involving thousands of patients with follow-up periods of more than 2 decades have demonstrated that local tumor control and disease-free survival (DFS) are comparable to that with radical mastectomy (4-10). In China, however, BCT was not used routinely until 2000, and far fewer patients choose it compared to those in Western countries. Thus, reports regarding its efficacy for Chinese women are few. We present here a large matched research of BCT compared to modified radical mastectomy (MRM) performed in China, and summarize the long-term follow-up results of BCT and MRM for breast cancer from a single unit, focusing on local recurrence (LR), DFS, and distant disease-free survival (DDFS) in the two groups.
Patients and methods
Study design
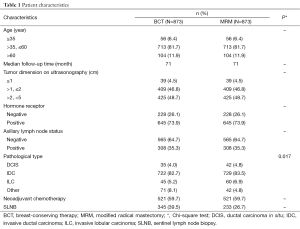
A consecutive series of women with primary breast cancer treated with BCT or MRM were derived from a database created and managed by Peking University Cancer Hospital & Institute. A total of 3,109 women were eligible for this study, of whom, 1,746 with stage I and II primary breast cancer were analyzed, and patients with stage III or IV breast cancer were excluded. BCS followed by radiotherapy to the intact breast was performed in 873 cases and MRM was performed in 873 cases between January 2000 and February 2009. These patients were matched according to 5 baseline variables which are thought to possibly have a significant association with LR, distant metastasis, and survival. These were age at diagnosis, axillary lymph node status, hormone receptor status, maximal diameter of the primary tumor, and the use of neoadjuvant chemotherapy (Table 1).
The match ratio was 1:1. The median patient age was 47 and 49 years in the BCT and MRM groups, respectively, and the median follow-up periods were 71 and 71 months, respectively. Neoadjuvant chemotherapy was administered to 521 patients (59.7%) in each group.
A cut-off date in February 2009 was chosen to give a minimum evaluable follow-up period of 41 months. Patients were clinically staged in accordance with American Joint Committee on Cancer (AJCC) guidelines (11).
Full table
Surgical procedures and other treatments
The surgical procedure included resection of the primary tumor with a surrounding margin of at least 1 cm of normal tissue, which was confirmed to be cancer-free through frozen pathological examination during the operation. Prior to 2004, all patients underwent axillary lymph node dissection (ALND). The surgical approach included level I, II, and partial level III ALND. At least 11 nodes were removed in each case. Sentinel lymph node biopsy (SLNB) was introduced in 2005. Patients with positive sentinel lymph nodes during this procedure went on to have a complete ALND. SLNB without complete dissection was performed in 578 patients with pathologically negative sentinel lymph nodes assessed by conventional and immunocytochemical staining.
After BCS, all patients were treated with radiotherapy using tangential fields directed at the intact breast. Radiotherapy was administered using standard techniques with 4-6 MeV linear accelerators. Patients received treatment to the whole breast at a standard fractionation of 2 Gy daily to a total median dose of 46 Gy (range, 40-60 Gy), followed by electron beam boost to the tumor bed to a total median dose of 60 Gy (range, 50-66 Gy) over a period of 6-8 weeks. Patients undergoing ALND received treatment to the breast alone if they had no more than 3 positive nodes pathologically or to the breast and supraclavicular and internal mammary nodes if there were ≥4 nodes positive pathologically. Patients undergoing SLNB were in general treated with tangents only if they were found to have pathologically negative sentinel nodes. Patients with positive sentinel nodes underwent complete ALND. The treatment policy for these patients was the same as that for those who had ALND initially. The principle of adjuvant (or neoadjuvant) chemotherapy and adjuvant hormonal therapy was in accordance with National Comprehensive Cancer Network/St. Gallen guidelines.
Hormone receptor and human epidermal growth factor receptor 2 expression
Data on estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER-2/neu) were obtained through standard clinical testing. ER and PR status were determined using immunohistochemistry (IHC) staining, and tumors were considered to be receptor-positive if more than 10% of cells stained positive. Tumors were considered HER-2-positive only if they had a +++ IHC score or a ++ IHC score and positive Her-2 gene amplification based on fluorescence in situ hybridization (FISH).
Definition of recurrence
The patients underwent annually mammography. Ultrasonography of bilateral breasts (or the remaining breast in cases of total mastectomy) and lymphatic regions were routinely performed every 3-6 months. Other diagnostic studies were performed at the discretion of the referring physician. Treatment failure was defined as histologically confirmed reappearance of cancer in the ipsilateral breast or chest wall. Regional nodal relapses were defined as clinical failure in the ipsilateral axilla, supraclavicular fossa, infraclavicular fossa, or internal mammary chain as the first site of failure. Patients with recurring breast carcinoma who also had positive nodes at the time of failure but no clinical signs of regional nodal failure were not considered to have experienced nodal relapse. Distant failure was defined as any clinical and/or radiographic evidence of metastatic disease.
Statistical analysis
The chi-square test was used to compare the distribution of baseline characteristics among subtypes. The probability of survival (or relapse occurrence), DFS, and DDFS were calculated using the Kaplan-Meier method. Statistical differences among LR, DFS, and DDFS rates were calculated using the log-rank test for univariate analysis. All tests were two-tailed. P<0.05 was considered statistically significant. Statistical tests were performed using SPSS 15.0 software package (SPSS Inc., Chicago, IL, USA).
Results
Local control
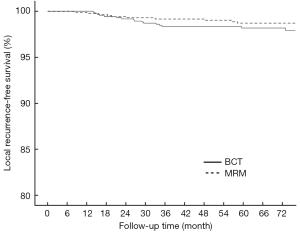
As of August 2013, with a minimum evaluable follow-up period of 41 months and a median follow-up period of 71 months, 17 cases of ipsilateral breast tumor recurrence (IBTR) have been noted in the BCT group and 10 cases of LR have been noted in the MRM group. Thus, the 6-year actuarial LR-free survival rates were 98.2% (95% CI: 0.973-0.989) and 98.7% (95% CI: 0.980-0.994), respectively. The incidence of LR was higher in the BCT group than in the MRM group at the 6th year (1.8% vs. 1.3%), but this difference was not statistically significant (P=0.182, Figure 1). Local regional relapses were noted in the axilla in 14 cases, in the supraclavicular fossa in 13 cases, and in the infraclavicular fossa in 1 case.
Impact of surgery type on DDFS and DFS
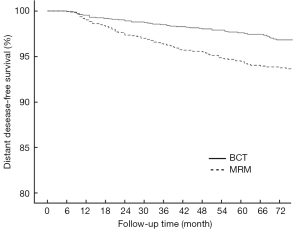
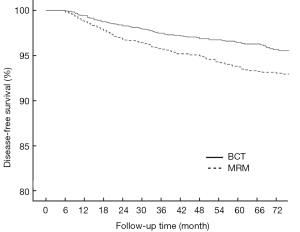
In the BCT and MRM groups, 51 and 109 patients, respectively, were found to have metastasis in distant organs and the 6-year DDFS was 93.6% (95% CI: 0.922-0.950) and 87.7% (95% CI: 0.854-0.900), respectively (P<0.001, Figure 2). The 6-year DFS rates in these groups were 91.3% (95% CI: 0.894-0.932) and 86.3% (95% CI: 0.840-0.886), respectively (P<0.001, Figure 3).
Subgroup analysis
We performed subgroup analysis to determine the occurrence of LR and distant metastasis in BCT patients according to clinical and pathological parameters (Table 2). The LR rate was significantly higher in patients with positive axillary lymph nodes (3.2%, 10 of 308) than in patients with negative axillary lymph nodes (1.2%, 7 of 565; P=0.030). The rates of actuarial breast relapse for T1 and T2 tumors were 1.1% (5 of 448) and 2.8% (12 of 425), respectively (P=0.067), and the incidence of LR was higher among patients who received neoadjuvant chemotherapy (2.7%, 14 of 521) than among those who did not receive neoadjuvant chemotherapy (0.9%, 3 of 352; P=0.062).
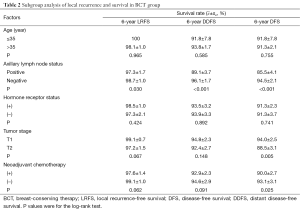
Full table
Discussion
Several previous retrospective and prospective randomized trials have shown that BCS followed by adjuvant radiotherapy is equivalent to mastectomy in terms of survival for patients with early stage breast cancer, despite of a higher rate of LR (2,12-14). Consequently, BCT has been used routinely in clinical practice for more than 20 years in many Western countries. The comparatively low take-up rate of BCT in China may relate to factors such as social and economic circumstances, although concern over the increased risk of relapse and metastasis seems to have been the primary consideration for both breast cancer patients and their doctors.
The study we report here has a longer follow-up period than any previous study conducted regarding the relative efficacy of BCT and MRM for primary breast carcinoma in China. In order to minimize the selection bias of patients between these groups, the data were analyzed retrospectively by a matched cohort study method using 5 baseline variables: patient age, axillary lymph node status, hormone receptor status, the use of neoadjuvant chemotherapy and tumor diameter. All these factors were considered likely to be associated with LR, distant metastasis, and survival, based on previous studies.
The ultimate aim of this study was to help patients and physicians in China decide whether BCT or MRM is the better option in any given case. We found that BCS followed by radiotherapy provides comparable results to those of MRM in terms of local control. This is consistent with the findings of earlier, randomized trials (1,2). One possible concern in interpreting these results is that IBTR may actually represent 2 distinct entities: a true recurrence (TR) and a new primary tumor (NPT). Some studies suggest that NPTs are associated with a more favorable outcome than TR (15-17). However, as no standard method for sub-classifying IBTR as either TR or NPT has been established yet, we did not attempt to distinguish these entities in this study.
Breast cancer has a prolonged natural history, and hence competing causes of mortality (for example heart disease and stroke) may potentially skew the DFS and DDFS data. We found that the BCT group had significantly better 6-year DFS and DDFS rates than the MRM group. These results are striking and suggest that BCT is likely to be the superior treatment option in most cases. This is consistent with the findings of several recent studies (18-20). However, because it is retrospective, this study is not sufficient to conclusively prove that BCT is superior to MRM. Unknown biases may have prevented us from identifying the true differences between the efficacy of BCT and MRM in these patients.
Some studies on the efficacy of BCT have found higher locoregional recurrence rates in younger patients (21-23). There was no such finding in a mastectomy series (24,25), and some investigators postulated that the higher locoregional recurrence rates were due to limited breast resection in younger patients (21). Younger age itself, however, was also shown to be associated with diverse, aggressive pathological features (23-26).
Neoadjuvant chemotherapy is an important method for enabling otherwise ineligible patients to undergo BCT (27,28). In this study, 59.7% of patients in each of the BCT and MRM groups received neoadjuvant chemotherapy. Within the BCT group, the 6-year DFS of patients who received neoadjuvant chemotherapy was significantly lower than that of patients who did not receive this treatment. This may reflect the fact that patients undergoing neoadjuvant chemotherapy did so because on average they had larger tumors and more positive axillary lymph nodes. Our subgroup analysis showed that both tumor size and axillary lymph node status at initial treatment are strongly associated with both the 6-year LRFS rate (lymph node status only) and the 6-year DFS and DDFS rates (lymph node status and tumor size). Tumors insensitive to neoadjuvant chemotherapy generally have more aggressive behavior, resulting in a poor prognosis for patients undergoing MRM.
We found statistical differences in the distribution of pathological types between the BCT and MRM groups in this study (P=0.017), suggesting that this potential selection bias might not be have been completely controlled. Previous studies have shown that invasive lobular carcinomas have similar rates of local control to invasive ductal carcinoma. Furthermore, the survival and local control afforded by BCT in patients with invasive lobular tumors do not differ statistically from those achieved in patients with invasive ductal tumors (29,30), which is consistent with this study.
HER-2 is an important prognostic marker of primary breast cancer. It was not included in this study, however, because HER-2 testing was not routine practice in our center until 2004, and subsequent HER-2 results were not of sufficient quality to allow the analysis. As compared with luminal A-like subtype, HER-2-positive was associated with a worse DFS in node-positive patients. Anti-HER-2 therapy results in a significant survival advantage when given after chemotherapy to early breast cancer patients over observation alone (31-33), and even to metastatic breast cancer patients (34). In the current study, only a few patients could afford trastuzumab treatment, but the inclusion of these few might also have biased the analysis of the results to some degree. The short follow-up period in our study also limits the comparison of BCT and MRM, and we hope that this might be addressed by future studies with an extended follow-up period.
Conclusions
Our matched retrospective study indicates that BCT performed for eligible patients is as effective as MRM with respect to local tumor control, DFS, and DDFS. BCT may be a superior treatment option for most Chinese primary breast cancer patients.
Acknowledgements
We would like to thank Mr. Yingjian He for his support and help with the statistical analysis.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Du X, Freeman DH Jr, Syblik DA. What drove changes in the use of breast conserving surgery since the early 1980s? The role of the clinical trial, celebrity action and an NIH consensus statement. Breast Cancer Res Treat 2000;62:71-9. [PubMed]
- Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002;347:1233-41. [PubMed]
- Kiebert GM, de Haes JC, van de Velde CJ. The impact of breast-conserving treatment and mastectomy on the quality of life of early-stage breast cancer patients: a review. J Clin Oncol 1991;9:1059-70. [PubMed]
- Atkins H, Hayward JL, Klugman DJ, et al. Treatment of early breast cancer: a report after ten years of a clinical trial. Br Med J 1972;2:423-9. [PubMed]
- Gasparini G, Panizzoni GA, Dal Fior S, et al. Conservative surgery and irradiation (QUART) in the treatment of 243 stage I-II breast cancer patients. Anticancer Res 1991;11:1635-40. [PubMed]
- Fisher B, Jeong JH, Anderson S, et al. Twenty-five-year follow-up of randomized trail comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med 2002;347:567-75. [PubMed]
- Jacobson JA, Danforth DN, Cowan KH, et al. Ten-year results of a comparison of conservation with mastectomy in the treatment of stage I and II breast cancer. N Engl J Med 1995;332:907-11. [PubMed]
- van Dongen JA, Voogd AC, Fentiman IS, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 Trail. J Natl Cancer Inst 2000;92:1143-50. [PubMed]
- Blichert-Toft M, Rose C, Andersen JA, et al. Danish randomized trail comparing breast conservation therapy with mastectomy: six years of life-table analysis. J Natl Cancer Inst Monogr 1992.19-25. [PubMed]
- Sarrazin D, Lê MG, Arriagada R, et al. Ten-year results of a randomized trial comparing a conservative treatment to mastectomy in early breast cancer. Radiother Oncol 1989;14:177-84. [PubMed]
- Singletary SE, Connolly JL. Breast cancer staging: working with the six edition of the AJCC Cancer Staging Manual. CA Cancer J Clin 2006;56:37-47. [PubMed]
- Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 2002;347:1227-32. [PubMed]
- Perez CA, Taylor ME, Halverson K, et al. Brachytherapy or electron beam boost in conservation therapy of carcinoma of the breast: a nonrandomized comparison. Int J Radiat Oncol Biol Phys 1996;34:995-1007. [PubMed]
- Litière S, Werutsky G, Fentiman IS, et al. Breast conserving therapy versus mastectomy for stage I-II breast cancer: 20 year follow-up of the EORTC 10801 phase 3 randomised trial. Lancet Oncol 2012;13:412-9. [PubMed]
- Smith TE, Lee D, Turner BC, et al. True recurrence vs. new primary ipsilateral breast tumor relapse: an analysis of clinical and pathologic differences and their implications in natural history, prognoses, and therapeutic management. Int J Radiat Oncol Biol Phys 2000;48:1281-9. [PubMed]
- Huang E, Buchholz TA, Meric F, et al. Classifying local disease recurrences after breast conservation therapy based on location and histology: new primary tumors have more favorable outcomes than true local disease recurrences. Cancer 2002;95:2059-67. [PubMed]
- Yoshida T, Takei H, Kurosumi M, et al. True recurrences and new primary tumors have different clinical features in invasive breast cancer patients with ipsilateral breast tumor relapse after breast-conserving treatment. Breast J 2010;16:127-33. [PubMed]
- Hwang ES, Lichtensztajn DY, Gomez SL, et al. Survival after lumpectomy and mastectomy for early stage invasive breast cancer: the effect of age and hormone receptor status. Cancer 2013;119:1402-11. [PubMed]
- Kurian AW, Lichtensztajn DY, Keegan TH, et al. Use of and mortality after bilateral mastectomy compared with other surgical treatments for breast cancer in California, 1998-2011. JAMA 2014;312:902-14. [PubMed]
- Agarwal S, Pappas L, Neumayer L, et al. Effect of breast conservation therapy vs mastectomy on disease-specific survival for early-stage breast cancer. JAMA Surg 2014;149:267-74. [PubMed]
- Zhou P, Recht A. Young age and outcome for women with early-stage invasive breast carcinoma. Cancer 2004;101:1264-74. [PubMed]
- Jones HA, Antonini N, Hart AA, et al. Impact of pathological characteristics on local relapse after breast-conserving therapy: a subgroup analysis of the EORTC boost versus no boost trial. J Clin Oncol 2009;27:4939-47. [PubMed]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Darby S, McGale P, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011;378:1707-16. [PubMed]
- Katz A, Strom EA, Buchholz TA, et al. Locoregional recurrence patterns after mastectomy and doxorubicin-based chemotherapy: implications for postoperative irradiation. J Clin Oncol 2000;18:2817-27. [PubMed]
- Zellars RC, Hilsenbeck SG, Clark GM, et al. Prognostic value of p53 for local failure in mastectomy-treated breast cancer patients. J Clin Oncol 2000;18:1906-13. [PubMed]
- van der Leest M, Evers L, van der Sangen MJ, et al. The safety of breast-conserving therapy in patients with breast cancer aged < or = 40 years. Cancer 2007;109:1957-64. [PubMed]
- Beriwal S, Schwartz GF, Komarnicky L, et al. Breast-conserving therapy after neoadjuvant chemotherapy: long-term results. Breast J 2006;12:159-64. [PubMed]
- Wolmark N, Wang J, Mamounas E, et al. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr 2001;96-102.
- Santiago RJ, Harris EE, Qin L, et al. Similar long-term results of breast-conservation treatment for stage I and II invasive lobular carcinoma compared with invasive ductal carcinoma of the breast: The University of Pennsylvania experience. Cancer 2005;103:2447-54. [PubMed]
- Vo TN, Meric-Bernstam F, Yi M, et al. Outcomes of breast-conservation therapy for invasive lobular carcinoma are equivalent to those for invasive ductal carcinoma. Am J Surg 2006;192:552-5. [PubMed]
- Gianni L, Dafni U, Gelber RD, et al. Treatment with trastuzumab for 1 year after adjuvant chemotherapy in patients with HER2-positive early breast cancer: a 4-year follow-up of a randomised controlled trial. Lancet Oncol 2011;12:236-44. [PubMed]
- Perez EA, Romond EH, Suman VJ, et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol 2014;32:3744-52. [PubMed]
- Joensuu H, Bono P, Kataja V, et al. Fluorouracil, epirubicin, and cyclophosphamide with either docetaxel or vinorelbine, with or without trastuzumab, as adjuvant treatments of breast cancer: final results of the FinHer Trial. J Clin Oncol 2009;27:5685-92. [PubMed]
- Yan M, Lv HM, Zhang MW, et al. Maintenance treatment of trastuzumab for patients with advanced breast cancer to achieve long term survival: two case reports and literature review. Chin J Cancer Res 2014;26:486-92. [PubMed]


