Nomogram for predicting lymph node metastasis rate of submucosal gastric cancer by analyzing clinicopathological characteristics associated with lymph node metastasis
Introduction
Gastric cancer is currently among the most common cancer worldwide and the second most common cause of cancer-related death (1,2). Early gastric cancer is defined as the lesion confined to the mucosa or submucosa, regardless of the size or the presence of regional lymph node metastasis (3,4). The treatment of early gastric cancer includes endoscopic surgery, wedge resection, laparoscopy-assisted gastrectomy and open gastrectomy (5-7). Endoscopic surgical techniques, including endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD), have been widely accepted as an alternate treatment for early gastric cancer patients without lymph node metastasis, which can preserve gastric function with low invasiveness (8-10). Endoscopic resection with curative intent is indicated only in tumors that fulfill the endoscopic resection criteria, because these tumors rarely metastasize to lymph nodes (11,12).
Although the incidence of lymph node metastasis is low in early gastric cancer, it is the most important prognostic factor for survival in resectable gastric adenocarcinoma (13,14). It is true that the rate of lymph node metastasis is much higher in submucosal tumors than in mucosal cancers, and the risk factors for nodal metastasis are quite different between the two groups (15). Therefore, preoperative prediction of lymph node metastasis is more important in the choice of treatment modality for submucosal gastric cancer patients. Accurate prediction of the risk of lymph node metastasis in submucosal gastric cancer is crucial because it can select patients suitable for endoscopic resection. Nomograms have been developed to quantify risk factors of lymph node metastasis in breast cancer and prostate cancer (16,17). However, there is no nomogram for predicting the risk of lymph node metastasis in submucosal gastric cancer. The aim of the present study was to identify risk factors for lymph node metastasis into a nomogram for patients with submucosal gastric cancer in order to guide management.
Materials and methods
Patients
The inclusion criteria were patients who underwent surgery achieving radical (R0) resection with D2 lymph node dissection and pathologically confirmed to be submucosal gastric cancer in accordance with the rules of the Japanese Gastric Cancer Association (JGCA). The exclusion criteria were patients who had preoperative therapy (18). Finally, a total number of 262 patients were involved in this study between December 1996 and December 2012. The clinicopathologic characteristics including gender, age and information on tumor size, depth of invasion, macroscopic type, histology, and the existence of lymphovascular invasion were retrieved from medical records. The maximum diameter of tumor was recorded as tumor size. The carcinomas were classified into three macroscopic types: protruding type (I), superficial type [II, including elevated (IIa), flat type (IIb), depressed type (IIc)], and excavated type (III). Tumor differentiation was divided into two groups: the differentiated group, which included well or moderately differentiated adenocarcinoma, and the undifferentiated group, which included poorly or undifferentiated carcinoma. Histological type was classified according to the World Health Organization (WHO) classification for gastric cancer, including adenocarcinoma, signet ring cell carcinoma, mucinous adenocarcinoma and so on. Lymph node involvement was pathologically confirmed postoperatively and classified according to the 7th edition of the Union for International Cancer Control (UICC) pN category (pN0: no metastasis; pN1: 1−2 metastatic lymph nodes; pN2: 3−6 metastatic lymph nodes; pN3: ≥7 metastatic lymph nodes). Resection margins were negative in all cases. This study was approved by the Institutional Review Board of the Beijing Cancer Hospital, and informed consent was obtained from all individuals.
Statistical analysis and construction of nomogram
All the statistical analyses and graphics were performed with the SPSS 20.0 statistical package (SPSS Inc., Chicago, IL, USA) and R version 2.11.1 (The R Foundation for Statistical Computing, Vienna, Austria). Associations between lymph node metastasis and clinicopathological parameters were analyzed using the chi-square test (or Fisher’s exact test when appropriate). The parameters that were of significance in the univariate analysis were selected as parameters in the logistic regression. A nomogram was developed as a tool for identifying patients at risk for lymph node metastasis, and it provided a graphical representation of the effect which can be used to calculate the risk of lymph node metastasis for an individual patient by the points associated with each risk factor (19,20). The predictive accuracy of the model was graphically displayed by the receiver operating characteristic (ROC) curve. Accuracy of the nomogram was then quantified using the area under the curve (AUC) for validation. Calibration was carried out for the constructed nomogram and internal validation was analyzed from 200 additional bootstrap samples to decrease the overfit bias and inspect the coincidence with the actual situation. Bootstrapping allows for the simulation of the performance of the nomogram if it is applied to future patients and provides an estimate of the average optimism of the AUC. The probability of lymph node metastasis was estimated with 95% confidence interval (95% CI) based on binominal distribution. P<0.05 was considered statistically significant.
Results
Correlations between lymph node metastasis and clinicopathological features in submucosal gastric cancer patients
The overall data from the 262 patients with submucosal gastric cancer were analyzed. The study included 180 males and 82 females, and the mean age was 58 years old (range, 24−82 years old). Among the submucosal gastric cancer patients, 48 (18.3%) were histologically shown to have lymph node metastasis including 34 (13.0%) with N1, 7 (2.7%) with N2, and 7 (2.7%) with N3, and 214 (81.7%) had no lymph node metastasis.
Lymph node metastasis was associated with age, tumor location, macroscopic type, size, differentiation, histology, and the existence of ulcer and lymphovascular invasion in submucosal gastric carcinoma (P<0.05). The patients younger than 50 years old have higher probability of lymph node metastasis than the elders (P=0.039). The tumor size larger than 2 cm was more likely to get lymph node metastasis than the smaller one (P=0.042). The macroscopic type I/II carcinomas have less possibility of lymph node metastasis than type III/mixed carcinomas (P=0.026). The differentiated carcinoma have low incidence of lymph node metastasis (P=0.014). Tumors with ulcer and lymphovascular invasion were association with higher lymph node metastasis (P=0.048, <0.001, respectively). The rate of lymph node metastasis in gastric adenocarcinoma was lower than other types (P=0.002). There was no significant difference between male and female in lymph node metastasis (Table 1).
The further multivariate logistic regression analysis showed that age ≤50 [P=0.026, relative risk (RR)=2.703, 95% CI: 1.126−6.494], macroscopic type III/mixed (P=0.020, RR=2.713, 95% CI: 1.169−6.295), undifferentiated type (P=0.008, RR=3.752, 95% CI: 1.404−10.028), and presence of lymphovascular invasion (P<0.001, RR=11.048, 95% CI: 4.831−25.266) were positively correlated to lymph node metastasis, suggesting that these characters were independent risk factors of lymph node metastasis in submucosal gastric cancer (Table 2).
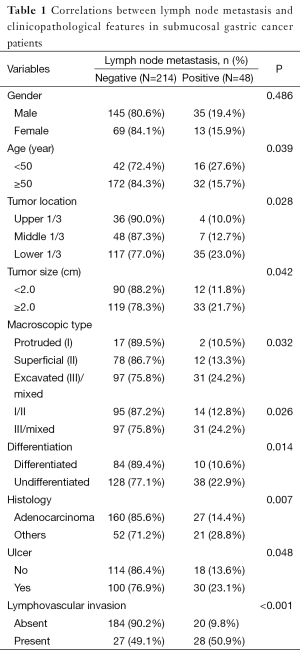
Full table
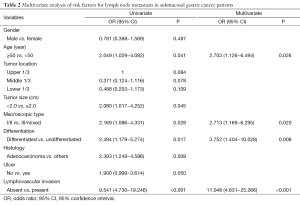
Full table
Nomogram for prediction of metastatic lymph nodes
Furthermore, we chose these 4 independent risk factors to develop a predicting nomogram for lymph node metastasis in submucosal gastric cancer patients (Figure 1). For each patient, points were assigned for each of these clinical variables (age, macroscopic type, differentiation, lymphovascular invasion), and the total score calculated from the nomogram corresponds to the predicted metastatic lymph node probability. After 200 repetitions of bootstrap sample corrections, we developed an ROC curve to estimate the predictive accuracy of the model which had an AUC of 0.844 (95% CI: 0.785−0.904) with a good calibration curve related to a mean error that never exceeded 5% implying good concordance (P<0.001) (Figure 2). The calibration curve in internal validation illustrates how the predictions of lymph node metastasis from the nomogram compare with the actual outcomes for all the submucosal gastric cancer patients, and the overall discrimination measurement of the validation cohort was 0.914 (Figure 3).

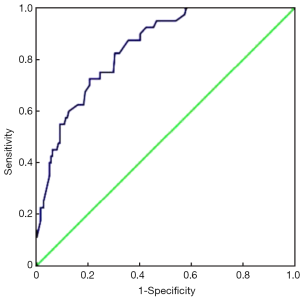
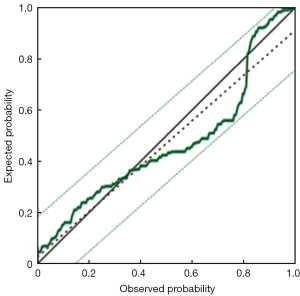
Discussion
The D2 lymphadenectomy (dissection of all group I and group II lymph nodes) was considered to be the standard and optimal surgical procedure for patients with early gastric cancer in past ten years (21,22). With the increased detection of early gastric cancer and development of endoscopic technology, the lymph node metastasis has become a matter of great concern. The lymph node metastasis rate of early gastric cancer is reported to be approximately 11% to 18%, and approximately 70% to 80% of patients will undergo overtreatment with D2 lymphadenectomy (8,22,23). Previous studies noted that depth of invasion was an important predictive factor of lymph node metastasis in early gastric cancer (24,25), and the probability of lymph node metastasis ranged from 10.2% to 22.9% in cases of submucosal gastric cancers, which was much higher than mucosal tumors (26,27). The gold standard in the curative treatment of gastric cancer is radical operation generally associated with D2 lymphadenectomy which has a high success rate in early cases (28). However, it is associated with some complications and mortality caused by this unnecessary procedure (29). And the postoperative complication rates of laparoscopy-assisted gastrectomy were 10−25% and major complication rates were 2−5% (30,31). Recently, endoscopic therapy has been attempted to preserve gastric function with low invasiveness, reduce the damage of excessive treatment and promote life quality in patients with submucosal gastric cancers, but lymph node dissection is beyond the capability of endoscopic therapy (32-36). That’s just the point, for submucosal gastric cancer patients with high operative risk, ESD could be considered under cautious criteria. The preoperative accurate prediction of the risk of lymph node metastasis is crucial because can select patients appropriate for endoscopic resection. Therefore, endoscopic therapy should only be used for submucosal gastric cancers if the risk of lymph node metastasis is negligible and a cure is expected after complete local resection.
Many cancer clinicians become more and more attracted to simple tools such as nomogram to improve the treatment of cancer. For this purpose, we analyzed retrospectively 262 submucosal gastric cancer patients based on clinical and routinely definitive pathological characteristics in order to find some clues for medical decision. The lymph node metastasis rate of all early gastric cancer patients is 18.3% in current study, which is similar to or lower than the previous results (37-39). The age, macroscopic type, differentiation and lymphovascular invasion were independent factors for lymph node metastasis, and the presence of lymphovascular invasion is considered to be the most important predictor for lymph node metastasis in submucosal gastric cancer, which was in agreement with the results of previous studies (11,12,37,40-43). Fidler reported that the initial step of the metastatic process is regional lymphatic-vascular invasion (44) and we also found that the presence of lymphovascular invasion is considered to be the most important predictor of lymph node metastasis.
Since lymph node metastasis in early gastric cancer is a rare event especially in mucosal gastric carcinoma, our investigation focused on submucosal tumors. Our study has provided a good and handy method to evaluate the risk of lymph node metastasis in submucosal gastric carcinoma. Based on our studies and the consistent previous studies, we choose all these independent characteristic features in our nomogram to predict the rate of lymph node metastasis in submucosal gastric cancer. The figure calculated from this nomogram could be employed to estimate the likelihood of metastatic lymph node involvement. The nomogram seems to be simple and practical with a relative high area under the ROC curve, with good performance related to a mean error that never exceeds 5%, which supported our selection of variables for determining suitable treatment. We quantified the gain in predictability on the basis of an increase in the area under the ROC curve. Internal validation demonstrated good discrimination power that the actual probability corresponds closely with the predicted probability. And all of these predictors can be relatively easy to collect clinically from pathologic examination of endoscopic resection specimen. According to the nomogram, certain subgroups of submucosal invasive gastric cancer can be followed up without additional surgical resection. For example, an undifferentiated and excavated (III)/mix type submucosal gastric cancer patient with lymphovascular invasion, who is younger than 50 years old, has more than 80% possibility of lymph node metastasis without considering other factors. This patient is extremely suitable for radical operation with lymphadenectomy, or laparoscopic lymph node dissection following endoscopic dissection. In contrast, another one with opposite characteristics: differentiated protruded/superficial (I/II) type submucosal cancer, without lymphovascular invasion, older than 50 years old, has almost no lymph node metastasis without respect to the others (much less than 5%), who is considered to select only less invasive endoscopic treatments. Moreover, we believe that our nomogram will assist surgeons in the selection of appropriate treatment for patients with submucosal gastric cancer regarding to the probability of the presence of lymph node metastasis.
As far as we know, this is the first study providing a nomogram to predict the rate of lymph node metastasis for submucosal gastric cancer. The potential limitations of this study should be considered that the cohort is small for low incidence, and we should enlarge the samples in order to improve the nomogram. This is a single center retrospective study which need for further external validation with different populations. Despite these limitations, this nomogram offers an effective tool to predict the rate of lymph node metastasis for submucosal gastric cancer patients, with which we could select patient who are suitable candidates for appropriated treatment.
Conclusions
In conclusion, the present study constructed a nomogram to predict the probability of lymph node metastasis in submucosal gastric cancer based on the data of age at diagnosis, macroscopic type, differentiation and lymphovascular invasion. This tool can assist clinicians and patients in quantifying the potential lymph node metastasis rate for making surgical decisions. Certain patients are suitable for radical operation or endoscopic dissection plus D2 lymphadenectomy, and others are selected for only ESD and EMR. For future study, we should expand the sample size, add different centers to prove this nomogram and determine the cutoff value of lymph node metastasis rate for different treatments.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Sim SH, Kim YJ, Oh DY, et al. The role of PET/CT in detection of gastric cancer recurrence. BMC Cancer 2009;9:73. [PubMed]
- Chen W, Zheng R, Zhang S, et al. Annual report on status of cancer in China, 2010. Chin J Cancer Res 2014;26:48-58. [PubMed]
- Katai H, Sano T. Early gastric cancer: concepts, diagnosis, and management. Int J Clin Oncol 2005;10:375-83. [PubMed]
- Bu Z, Ji J. Controversies in the diagnosis and management of early gastric cancer. Chin J Cancer Res 2013;25:263-6. [PubMed]
- Kitano S, Shiraishi N, Uyama I, et al. A multicenter study on oncologic outcome of laparoscopic gastrectomy for early cancer in Japan. Ann Surg 2007;245:68-72. [PubMed]
- Soetikno R, Kaltenbach T, Yeh R, et al. Endoscopic mucosal resection for early cancers of the upper gastrointestinal tract. J Clin Oncol 2005;23:4490-8. [PubMed]
- Bonenkamp JJ, Hermans J, Sasako M, et al. Extended lymph-node dissection for gastric cancer. N Engl J Med 1999;340:908-14. [PubMed]
- Hyung WJ, Cheong JH, Kim J, et al. Application of minimally invasive treatment for early gastric cancer. J Surg Oncol 2004;85:181-5; discussion 186. [PubMed]
- Gotoda T, Jung HY. Endoscopic resection (endoscopic mucosal resection/endoscopic submucosal dissection) for early gastric cancer. Dig Endosc 2013;25 Suppl 1:55-63. [PubMed]
- Ishikawa S, Togashi A, Inoue M, et al. Indications for EMR/ESD in cases of early gastric cancer: relationship between histological type, depth of wall invasion, and lymph node metastasis. Gastric Cancer 2007;10:35-8. [PubMed]
- Isomoto H, Shikuwa S, Yamaguchi N, et al. Endoscopic submucosal dissection for early gastric cancer: a large-scale feasibility study. Gut 2009;58:331-6. [PubMed]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric cancer 2011;14:113-23. [PubMed]
- Kim JP, Kim YW, Yang HK, et al. Significant prognostic factors by multivariate analysis of 3926 gastric cancer patients. World J Surg 1994;18:872-7. [PubMed]
- Siewert JR, Böttcher K, Stein HJ, et al. Relevant prognostic factors in gastric cancer: ten-year results of the German Gastric Cancer Study. Ann Surg 1998;228:449-61. [PubMed]
- Lim MS, Lee HW, Im H, et al. Predictable factors for lymph node metastasis in early gastric cancer-analysis of single institutional experience. J Gastrointest Surg 2011;15:1783-8. [PubMed]
- Klar M, Jochmann A, Foeldi M, et al. The MSKCC nomogram for prediction the likelihood of non-sentinel node involvement in a German breast cancer population. Breast Cancer Res Treat 2008;112:523-31. [PubMed]
- Briganti A, Larcher A, Abdollah F, et al. Updated nomogram predicting lymph node invasion in patients with prostate cancer undergoing extended pelvic lymph node dissection: the essential importance of percentage of positive cores. Eur Urol 2012;61:480-7. [PubMed]
- Santiago JM, Sasako M, Osorio J. TNM-7th edition 2009 (UICC/AJCC) and Japanese Classification 2010 in Gastric Cancer. Towards simplicity and standardisation in the management of gastric cancer. Cir Esp 2011;89:275-81.
- Wang SJ, Lemieux A, Kalpathy-Cramer J, et al. Nomogram for predicting the benefit of adjuvant chemoradiotherapy for resected gallbladder cancer. J Clin Oncol 2011;29:4627-32. [PubMed]
- Luomaranta A, Leminen A, Loukovaara M. Prediction of lymph node and distant metastasis in patients with endometrial carcinoma: a new model based on demographics, biochemical factors, and tumor histology. Gynecol Oncol 2013;129:28-32. [PubMed]
- Borie F, Plaisant N, Millat B, et al. Appropriate gastric resection with lymph node dissection for early gastric cancer. Ann Surg Oncol 2004;11:512-7. [PubMed]
- Nitti D, Marchet A, Mammano E, et al. Extended lymphadenectomy (D2) in patients with early gastric cancer. Eur J Surg Oncol 2005;31:875-81. [PubMed]
- Roviello F, Rossi S, Marrelli D, et al. Number of lymph node metastases and its prognostic significance in early gastric cancer: a multicenter Italian study. J Surg Oncol 2006;94:275-80. [PubMed]
- Park DJ, Lee HK, Lee HJ, et al. Lymph node metastasis in early gastric cancer with submucosal invasion: feasibility of minimally invasive surgery. World J Gastroenterol 2004;10:3549-52. [PubMed]
- Jin EH, Lee DH, Jung SA, et al. Clinicopathologic factors and molecular markers related to lymph node metastasis in early gastric cancer. World J Gastroenterol 2015;21:571-7. [PubMed]
- Son HJ, Song SY, Kim S, et al. Characteristics of submucosal gastric carcinoma with lymph node metastatic disease. Histopathology 2005;46:158-65. [PubMed]
- Nonaka S, Oda I, Nakaya T, et al. Clinical impact of a strategy involving endoscopic submucosal dissection for early gastric cancer: determining the optimal pathway. Gastric Cancer 2011;14:56-62. [PubMed]
- Sano T, Aiko T. New Japanese classifications and treatment guidelines for gastric cancer: revision concepts and major revised points. Gastric Cancer 2011;14:97-100. [PubMed]
- Sasako M. Risk factors for surgical treatment in the Dutch Gastric Cancer Trial. Br J Surg 1997;84:1567-71. [PubMed]
- Ahn SH, Lee JH. Comparative study of clinical outcomes between laparoscopy-assisted proximal gastrectomy (LAPG) and laparoscopy-assisted total gastrectomy (LATG) for proximal gastric cancer. Gastric Cancer 2013;16:282-9. [PubMed]
- Kim HH, Hyung WJ, Cho GS, et al. Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report--a phase III multicenter, prospective, randomized Trial (KLASS Trial). Ann Surg 2010;251:417-20. [PubMed]
- Oka S, Tanaka S, Kaneko I, et al. Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest Endosc 2006;64:877-83. [PubMed]
- Choi IJ, Lee JH, Kim YI, et al. Long-term outcome comparison of endoscopic resection and surgery in early gastric cancer meeting the absolute indication for endoscopic resection. Gastrointest Endosc 2015;81:333-41. [PubMed]
- Miyamoto S, Muto M, Hamamoto Y, et al. A new technique for endoscopic mucosal resection with an insulated-tip electrosurgical knife improves the completeness of resection of intramucosal gastric neoplasms. Gastrointest Endosc 2002;55:576-81. [PubMed]
- Probst A, Pommer B, Golger D, et al. Endoscopic submucosal dissection in gastric neoplasia - experience from a European center. Endoscopy 2010;42:1037-44. [PubMed]
- Imagawa A, Okada H, Kawahara Y, et al. Endoscopic submucosal dissection for early gastric cancer: results and degrees of technical difficulty as well as success. Endoscopy 2006;38:987-90. [PubMed]
- An JY, Baik YH, Choi MG, et al. Predictive factors for lymph node metastasis in early gastric cancer with submucosal invasion: analysis of a single institutional experience. Ann Surg 2007;246:749-53. [PubMed]
- Hölscher AH, Drebber U, Mönig SP, et al. Early gastric cancer: lymph node metastasis starts with deep mucosal infiltration. Ann Surg 2009;250:791-7. [PubMed]
- Park YD, Chung YJ, Chung HY, et al. Factors related to lymph node metastasis and the feasibility of endoscopic mucosal resection for treating poorly differentiated adenocarcinoma of the stomach. Endoscopy 2008;40:7-10. [PubMed]
- Gotoda T, Yanagisawa A, Sasako M, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer 2000;3:219-225. [PubMed]
- Lee SH, Jee SR, Kim JH, et al. Intramucosal gastric cancer: the rate of lymph node metastasis in signet ring cell carcinoma is as low as that in well-differentiated adenocarcinoma. Eur J Gastroenterol Hepatol 2015;27:170-4. [PubMed]
- Hanaoka N, Tanabe S, Mikami T, et al. Mixed-histologic-type submucosal invasive gastric cancer as a risk factor for lymph node metastasis: feasibility of endoscopic submucosal dissection. Endoscopy 2009;41:427-32. [PubMed]
- Sanomura Y, Oka S, Tanaka S, et al. Predicting the absence of lymph node metastasis of submucosal invasive gastric cancer: expansion of the criteria for curative endoscopic resection. Scand J Gastroenterol 2010;45:1480-7. [PubMed]
- Fidler IJ. Critical factors in the biology of human cancer metastasis: twenty-eighth G.H.A. Clowes memorial award lecture. Cancer Res 1990;50:6130-8. [PubMed]


