Molecular aspects of prostate cancer with neuroendocrine differentiation
Introduction
Prostate cancer is one of the most common malignancies in men worldwide with variable incidence. In Asian countries, such as in China, South Korea, India, Japan, Thailand, Philippines, and Vietnam, the incidence of prostate cancer is much lower, compared with that in western countries (1,2). However, the incidence has increased about 12−14% in China annually (1). Patients with prostate cancer are currently treated mainly based on tumor grade and stage. Most patients have indolent disease and just need active surveillance. As a standard therapy strategy, androgen-deprivation therapy (ADT) is widely used for the treatment of advanced and metastatic prostate cancer. Many patients receiving androgen-deprivation may experience disease regression for a while, but nearly all of them will eventually progress to castration-resistant prostate cancer (CRPC) with no response to hormonal therapy. More and more studies are carried out to try to find the underlying mechanism of the development of CRPC during the past several decades. There are several theories proposed, most regarding androgen receptor (AR). One of the diverse mechanisms is that prostate cancer cells will undergo neuroendocrine differentiation (NED) after the patients have received hormonal therapy, which will inhibits androgen production or blocks AR function. This mechanism is believed to be significantly associated with the development of CRPC.
Recently, Epstein et al. have proposed a novel morphologic classification of prostate cancer with NED (3). The proposed classification is as following: (I) usual prostate adenocarcinoma with NED; (II) adenocarcinoma with Paneth cell-like NED; (III) carcinoid tumor; (IV) small cell carcinoma; (V) large cell NE carcinoma; (VI) mixed NE carcinoma-acinar adenocarcinoma. NED is not uncommon in prostate cancer. The proposed classification is more complicated when compared with the 2004 WHO classification (Table 1). It is about 10% of usual prostate adenocarcinoma shows focal NED detected by immunohistochemical staining with different markers, such as synaptophysin (Syn), chromogranin A (CgA) and CD56 (4,5). The prognostic significance of focal NED of usual prostate adenocarcinoma remains controversial. Most of the studies have shown no effect of NED on patients’ outcome (6,7). However, prostate cancers treated with androgen-deprivation usually show an increased NED and sometimes progress to secondary small cell carcinoma of prostate cancer with very poor outcome.
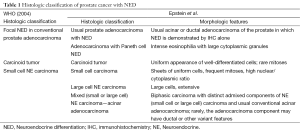
Full table
Neuroendocrine cells in normal prostate
Neuroendocrine cells are found in many tissues including normal prostate. NE cells in normal prostate, though a small subset of cells, are randomly interspersed amongst the luminal and basal cells of the prostate glands in all anatomic zones, with a slight more cells in transitional zone and peripheral zone than that in central zone (8). They are not readily recognized under the light microscope using conventional hematoxylin and eosin staining, but can be easily demonstrated by immunohistochemical staining with specific markers, such as Syn, CgA and CD56 etc. Under electron microscope, there are two different morphologic types of NE cells: the open-type cells and the closed-type cells (5). The open-type cells possess long surface microvilli through which the cells reach the lumen and receive luminal stimuli (pH, chemicals). The closed-type cells have lateral processes like dendritic cells through which the cells can contact the adjacent epithelial cells (luminal cells and basal cells), and receive stimuli from nerve endings, neighboring blood vessels and underlying stromal cells. The different morphologic types of NE cells are found to distribute differently in the prostate and seminal vesicles when the topography and structure of the excretory ducts of the different glands are analyzed in male rats (9). Approximately 40% of the NE cells of the ventral prostate ducts are of the open-type, whereas 14% of the seminal vesicle ducts, where most of the NE cells are of the closed-type (9). The finding suggests that the distribution pattern and different morphologic types of NE cells may be associated with different function (10).
As part of a diffuse NE system, NE cells in the prostate secrete a variety of peptide hormones, such as CgA, neuron-specific enolase (NSE), Syn, neural growth factor (NGF), bombesin/gastrin-releasing peptide, serotonin, histamine, calcitonin and other members of the calcitonin gene family, neuropeptide Y, vasoactive intestinal peptide, parathyroid hormone-related protein, somatostatin, vascular endothelial growth factor, and others. These substances regulate prostatic cells both in benign and malignant conditions endocrine, paracrine, and autocrine mechanisms (11). NE cells in the prostate are immunohistochemically positive for CgA, Syn, and CD56 etc., but negative for prostate specific antigen (PSA), AR and Ki67 because they appear to be non-proliferative and postmitotic cells.
NED in prostate cancer
As mentioned above, it is about 10% of untreated usual prostate adenocarcinoma shows focal NED (4,5). Focal NED shows such a highly heterogeneous phenomenon in prostate cancer, not only because of the true inherent heterogeneity of prostate cancer, but also because of the variable number of blocks detected and variable number of antibodies used. It is still remains uncertain whether patients of typical adenocarcinomas with focal NED have worse prognosis when compared with those without focal NED. It is not recommended to routinely use immunohistochemical stains to detect any NED in an morphologically typical primary adenocarcinoma of the prostate, particularly at early prostate cancer stage (3,12). The focal NED cells usually show the same appearance as the usual prostate adenocarcinoma; not necessarily resembling normal NE cells (13,14).
De novo NE tumors of the prostate, which are composed of exclusive NE tumor cells without history of prostate adenocarcinoma, are very rare. The tumors that fit in this category include carcinoid tumor, small cell carcinoma, and large cell NE carcinoma of the prostate. These tumors can be pure or admixed with prostatic adenocarcinoma. Carcinoid tumor is an extremely rare tumor with classic morphology at other sites, without close relation to usual prostate carcinoma or extension from bladder or urethra (3). It is a well-differentiated NE tumor with relatively good prognosis. It is suggested that carcinoid tumor can be subclassified according to mitotic rates and Ki67 proliferation rates as the criteria used for those of the gastrointestinal tract. Unlike usual prostate adenocarcinoma with focal NED, small cell carcinoma and large cell NE carcinoma of the prostate are very aggressive tumors with median cancer-specific survival of less than 2 years and 7 months, respectively (15,16). NE cells in prostate cancer are different from normal prostate NE cells in their protein expression (17). There are several hypotheses (18,19): (I) transformation of multi-potential prostatic progenitors; (II) transformation of normal prostatic NE cells; (III) transformation of prostate adenocarcinoma.
NED in prostate cancer increases after androgen deprivation and in CRPC (20). The mechanisms are not very clear regarding how the prostate carcinoma cells undergo NED when the tumor is treated with androgen deprivation. A number of experimental data generated in vitro found that multiple signaling pathways might contribute to NED (4), such as signal transducer and activator of transcription-3 (STAT-3), mitogen activated protein kinases (MAPKs), cyclic AMP-dependent protein kinase (PKA) and phosphatidylinositol 3-kinase (PI3K) dependent signaling pathways, in which phosphatidylinositol 3-kinase-AKT-mammalian target of rapamycin pathway (PI3K-AKT-mTOR) is essential for NED in prostate cancer (PCa) (21). Wnt pathway also plays a role in NED of prostate cancer other than the pathways mentioned above. All these pathways form a network and interact with each other to participate in the process of NED in prostate cancer.
There are many biomarkers used to detect NE component, such as CgA, Syn, NSE, and CD56. These antibodies are not only diagnostic ancillary tools, but also act as screening biomarkers with prognostic significance. Despite its name as neuron specific enolase, NSE immunoreactivity is not sufficiently specific for the diagnosis of NED (3). CgA, also known as secretory protein I, is an acidic protein encoded by the CHGA gene in humans (22,23). CgA was initially isolated as the major soluble protein of adrenal medullary chromaffin granules (24). This highly acidic protein is co-stored and co-released from storage vesicles along with catecholamines upon neural stimulation of the adrenal gland, or with the parathyroid hormone in response to hypocalcemia in the parathyroid gland (25,26). The localization of CgA has stimulated interest in CgA as a marker for differentiation of normal and neoplastic tissues of the diffuse endocrine and neuroendocrine system or cancer cells that can undergo NED. Elevated CgA expression levels correlated with disease burden and poor outcomes of prostate cancer (27,28). CgA appears to be the most sensitive marker and is most widely used for detecting NED either at the tissue level or in the general circulation (29-32). The availability of such a specific marker for the NE component could assist in identifying and monitoring NE cells in prostate cancer. Syn, also known as P38, is an acidic calcium binding glycoprotein closely associated with synaptic structure and function, and is an integral membrane protein of synaptic vesicles (33). It has been reported that the expression level of Syn is higher in malignant prostate tissue compared with that in benign tissue (34,35). Furthermore, high Syn levels are associated with poor survival of the patients with prostate carcinoma metastasis to bone (36). CD56, also known as neural-cell adhesion molecule (NCAM), is a membranous marker implicated into the intercellular adhesion that plays a key role in the cell-to-cell interactions well as cell-matrix interactions during development and differentiation of the nervous system. It is the most sensitive antibody especially in lung endocrine cancers. It has been reported that the sensitivity of this antibody reach up to 90−100% (37,38).
Molecular components driving NED in prostate cancer
In the last years, the molecular components involved in the NE differentiation of prostate cancer have been highly studied. Several molecular signatures have been implicated in prostate cancer with NED (Table 2).
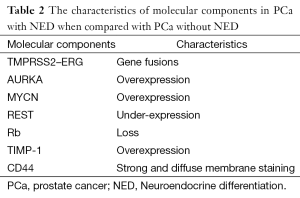
Full table
Transmembrane protease serine 2 (TMPRSS2) is an androgen-regulated, type II transmembrane-bound serine protease expresses in the prostate and overexpresses in neoplastic prostate epithelium. The rearrangement occurs between TMPRSS2 and an ETS transcription factor family member, most common early growth response proteins, ERG (39,40). ERG is a family of zinc finger transcription factors that includes Egr1, Egr2, Egr3 and Egr4. These four proteins show high degree of homology at their DNA-binding zinc finger domains, but they have divergent sequences outside the DNA-binding domains. All four EGR proteins bind to a 9-bp GC-rich DNA sequence called ERE that is found in multiple target gene promoters (41). The fusion of TMPRSS2-ERG results in an aberrant function of the ERG oncogene in cancer cells and consequently tumor progression in prostate cancer (42,43). Studies have documented the occurrence of TMPRSS2-ERG gene fusions in androgen-independent metastases of prostatic small cell carcinomas (44). Furthermore, several studies reported that TMPRSS2-ERG rearrangements were present in primary prostatic small cell carcinomas (45,46), which made it possible to distinguish prostatic small cell carcinoma from other small cell carcinomas such as metastasis or local extension from other sites such as the bladder using fluorescence in situ hybridization (FISH) or reverse transcription polymerase chain reaction of TMPRSS2-ERG gene fusion rearrangement.
The cell cycle kinase AURKA and MYCN are found to be overexpressed in prostate cancer with NED when compared with prostate adenocarcinoma by next-generation sequencing (47). The aurora kinase family includes aurora-A, -B and -C in mammals, encoded by AURKA gene mapped to chromosome 20q13.2 and initially discovered in 1995 (48). Aurora-A is a more extensively studied member of the aurora kinase family. Aurora-A predominantly localizes to the centrosome, and regulates centrosome maturation, entry into mitosis, formation and function of the bipolar spindle, and cytokinesis (49). As for MYCN, it is a member of the MYC family and encodes a transcription factor, N-myc. Aurora-A is known to stabilize N-myc, and gene amplification of AURKA and MYCN plays a leading role in the pathogenesis of malignancy for many types of tumor (50,51). Beltran et al. showed that overexpression of AURKA and MYCN were present in about 65% of primary prostate adenocarcinoma specimens that subsequently developed NEPC after ADT and in approximately 90% of the corresponding metastatic samples (47). Conversely, the same amplifications were only appearing in 5% of 169 unselected prostate adenocarcinoma tumors (52). To investigate the mechanism of Aurora kinase A or N-myc in NED, Beltran et al. assessed neuroendocrine marker expression after overexpression of AURKA and MYCN in RWPE-1 and LNCaP. The results showed that either AURKA or MYCN induced the expression of the neuroendocrine markers Syn and NSE, which are not normally expressed in benign prostate. Furthermore, inhibition of AURKA suppressed NSE expression in the NEPC cell line NCI-H660. Chromatin immunoprecipitation assay demonstrated that N-myc binds the promoters of NSE, Syn, and AR, suggesting direct modulation of the neuroendocrine phenotype (47). These results indicated that AURKA and MYCN could play important roles in the development of neuroendocrine carcinoma of the prostate and also suggested these molecular alterations may predispose for development of the NED in prostate cancer.
Another important finding is that RE-1 silencing transcription factor (REST) was down-regulated in association with the prevalence of NE phenotype in prostate cancer (53). The REST or neuron-restrictive silencer factor (NRSF) was identified in 1995 as a master transcriptional repressor with a critical role in suppressing expression of neuronal-specific genes (54,55). REST has been demonstrated to play crucial roles during embryonic development and neurogenesis. In addition, as REST plays important roles in the regulation of gene expression, abnormal function of REST has been associated with NE phenotype (56). Studies show that REST gene was down-regulated in 50% of NEPC and hybrid PC/NEPC tumors. Meanwhile, the neuroendocrine phenotype genes, harboring experimentally validated REST binding site, were up-regulated. In contrast, only 3% of other tumors from the same cohort exhibited this pattern (53,57). In addition, REST knockdown by siRNA leads to increased NED, and immunofluorescence analysis shows that CgA-positive cells are negative for nuclear REST staining, suggesting that nuclear REST functions as a transcriptional repressor that inhibits NED (53,58). Studies demonstrated that chronically androgen deprivation as well as AR blockage with antagonist MDV3100 leads to the reduced expression of REST coinciding with increased levels of CgA. In addition, REST protein downregulation, either directly by siRNA knockdown or indirectly through AR inhibition, leads to increased NED, suggesting that one of the functions of REST in NED is by the androgen/AR axis (58).
Some studies show that Rb loss underlies the development of NED in prostate cancer (59). Rb is encoded by the human retinoblastoma gene RB1 that was initially cloned from children with a rare form of eye cancer of the same name. RB is a negative regulator of the cell cycle and has been found to be inactivated in a wide range of human cancers (60). By immunohistochemistry (IHC) assay, studies showed that loss of Rb occurred almost universally in prostatic small cell carcinomas. In contrast, loss of this tumor suppressor occurs rarely in conventional high-grade acinar prostate tumors, acinar carcinomas with NED, and castrate-resistant prostate carcinomas (59,61). These data suggest that Rb loss is a critical event in the development of small cell carcinomas and may be a useful diagnostic and potential therapeutic target.
As for TIMP-1, an inhibitor of metalloproteinases and has been implicated in the development and progression of multiple types of tumors (62-64). Gong et al. published their results on the relationship between the circulating TIMP-1 levels and NED of prostate cancer. They found that circulating TIMP-1 levels were elevated in CRPC patients and that this elevation was associated with higher CgA levels and lower PSA levels in blood. Moreover, results from tissue and cell line studies were consistent with this concept that elevated TIMP-1 expression in CRPC was associated with NED (65). These data reveal that the tissue level or the general circulation of TIMP-1 may be a potential biomarker for patients with NEPC.
Furthermore, CD44, a major cell-surface receptor for hyaluronic acid and mediates epithelial cell adhesion by its involvement in cell-cell and cell-matrix interactions, has been shown strong and diffuse membrane staining by IHC in 100% of the prostatic small cell carcinoma, whereas in usual prostatic adenocarcinomas only rare positive scattered tumor cells are CD44 positive (66,67).
Conclusions
During the past decade, more and more data show that NED in prostate cancer contributes to the resistance to ADT therapy and cancer progression to CRPC. Immunohistochemical staining for NE cell markers such as Syn, CgA and CD56 can detect NED in prostate cancer, and theses markers can also predict the oncologic outcomes in patients at CRPC stage. There are variable molecular changes involved in NED of prostate cancer after ADT, and many molecules may contribute to the process of NED. The gene profiling may help to elucidate the complicate mechanisms in this process and help researchers find new and efficient target for postponing or even preventing NED of prostate cancer after ADT, from which the patients with prostate cancer will benefit.
Acknowledgements
Funding: This work was supported by the National Natural Science Foundation of China (81502244).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Center MM, Jemal A, Lortet-Tieulent J, et al. International variation in prostate cancer incidence and mortality rates. Eur Urol 2012;61:1079-92. [PubMed]
- Kimura T. East meets West: ethnic differences in prostate cancer epidemiology between East Asians and Caucasians. Chin J Cancer 2012;31:421-9. [PubMed]
- Epstein JI, Amin MB, Beltran H, et al. Proposed morphologic classification of prostate cancer with neuroendocrine differentiation. Am J Surg Pathol 2014;38:756-67. [PubMed]
- Komiya A, Suzuki H, Imamoto T, et al. Neuroendocrine differentiation in the progression of prostate cancer. Int J Urol 2009;16:37-44. [PubMed]
- Vashchenko N, Abrahamsson PA. Neuroendocrine differentiation in prostate cancer: implications for new treatment modalities. Eur Urol 2005;47:147-55. [PubMed]
- Jeetle SS, Fisher G, Yang ZH, et al. Neuroendocrine differentiation does not have independent prognostic value in conservatively treated prostate cancer. Virchows Arch 2012;461:103-7. [PubMed]
- Shariff AH, Ather MH. Neuroendocrine differentiation in prostate cancer. Urology 2006;68:2-8. [PubMed]
- Santamaría L, Martín R, Martín JJ, et al. Stereologic estimation of the number of neuroendocrine cells in normal human prostate detected by immunohistochemistry. Appl Immunohistochem Mol Morphol 2002;10:275-81. [PubMed]
- Aumüller G, Doll A, Wennemuth G, et al. Regional distribution of neuroendocrine cells in the urogenital duct system of the male rat. Prostate 2012;72:326-37. [PubMed]
- Montuenga LM, Guembe L, Burrell MA, et al. The diffuse endocrine system: from embryogenesis to carcinogenesis. Prog Histochem Cytochem 2003;38:155-272. [PubMed]
- Long RM, Morrissey C, Fitzpatrick JM, et al. Prostate epithelial cell differentiation and its relevance to the understanding of prostate cancer therapies. Clin Sci (Lond) 2005;108:1-11. [PubMed]
- Surcel CI, van Oort IM, Sooriakumaran P, et al. Prognostic effect of neuroendocrine differentiation in prostate cancer: A critical review. Urol Oncol 2015;33:265.e1-7.
- Nakada SY, di Sant'Agnese PA, Moynes RA, et al. The androgen receptor status of neuroendocrine cells in human benign and malignant prostatic tissue. Cancer Res 1993;53:1967-70. [PubMed]
- Sauer CG, Roemer A, Grobholz R. Genetic analysis of neuroendocrine tumor cells in prostatic carcinoma. Prostate 2006;66:227-34. [PubMed]
- Deorah S, Rao MB, Raman R, et al. Survival of patients with small cell carcinoma of the prostate during 1973-2003: a population-based study. BJU Int 2012;109:824-30. [PubMed]
- Evans AJ, Humphrey PA, Belani J, et al. Large cell neuroendocrine carcinoma of prostate: a clinicopathologic summary of 7 cases of a rare manifestation of advanced prostate cancer. Am J Surg Pathol 2006;30:684-93. [PubMed]
- Sun Y, Niu J, Huang J. Neuroendocrine differentiation in prostate cancer. Am J Transl Res 2009;1:148-62. [PubMed]
- Yao JL, Madeb R, Bourne P, et al. Small cell carcinoma of the prostate: an immunohistochemical study. Am J Surg Pathol 2006;30:705-12. [PubMed]
- Hu Y, Ippolito JE, Garabedian EM, et al. Molecular characterization of a metastatic neuroendocrine cell cancer arising in the prostates of transgenic mice. J Biol Chem 2002;277:44462-74. [PubMed]
- Hirano D, Okada Y, Minei S, et al. Neuroendocrine differentiation in hormone refractory prostate cancer following androgen deprivation therapy. Eur Urol 2004;45:586-92; discussion 592. [PubMed]
- Wu C, Huang J., et al. Phosphatidylinositol 3-kinase-AKT-mammalian target of rapamycin pathway is essential for neuroendocrine differentiation of prostate cancer. J Biol Chem 2007;282:3571-83. [PubMed]
- Simon JP, Aunis D. Biochemistry of the chromogranin A protein family. Biochem J 1989;262:1-13. [PubMed]
- Helman LJ, Ahn TG, Levine MA, et al. Molecular cloning and primary structure of human chromogranin A (secretory protein I) cDNA. J Biol Chem 1988;263:11559-63. [PubMed]
- Blaschko H, Comline RS, Schneider FH, et al. Secretion of a chromaffin granule protein, chromogranin, from the adrenal gland after splanchnic stimulation. Nature 1967;215:58-9. [PubMed]
- Winkler H. The composition of adrenal chromaffin granules: an assessment of controversial results. Neuroscience 1976;1:65-80. [PubMed]
- Fasciotto BH, Denny JC, Greeley GH Jr, et al. Processing of chromogranin A in the parathyroid: generation of parastatin-related peptides. Peptides 2000;21:1389-401. [PubMed]
- Burgio SL, Conteduca V, Menna C, et al. Chromogranin A predicts outcome in prostate cancer patients treated with abiraterone. Endocr Relat Cancer 2014;21:487-93. [PubMed]
- Conteduca V, Burgio SL, Menna C, et al. Chromogranin A is a potential prognostic marker in prostate cancer patients treated with enzalutamide. Prostate 2014;74:1691-6. [PubMed]
- Berruti A, Bollito E, Cracco CM, et al. The prognostic role of immunohistochemical chromogranin a expression in prostate cancer patients is significantly modified by androgen-deprivation therapy. Prostate 2010;70:718-26. [PubMed]
- Berruti A, Dogliotti L, Mosca A, et al. Circulating neuroendocrine markers in patients with prostate carcinoma. Cancer 2000;88:2590-7. [PubMed]
- Matei DV, Renne G, Pimentel M, et al. Neuroendocrine differentiation in castration-resistant prostate cancer: a systematic diagnostic attempt. Clin Genitourin Cancer 2012;10:164-73. [PubMed]
- Sciarra A, Di Silverio F, Autran AM, et al. Distribution of high chromogranin A serum levels in patients with nonmetastatic and metastatic prostate adenocarcinoma. Urol Int 2009;82:147-51. [PubMed]
- Wiedenmann B, Franke WW. Identification and localization of synaptophysin, an integral membrane glycoprotein of Mr 38,000 characteristic of presynaptic vesicles. Cell 1985;41:1017-28. [PubMed]
- Bonkhoff H. Neuroendocrine differentiation in human prostate cancer. Morphogenesis, proliferation and androgen receptor status. Ann Oncol 2001;12 Suppl 2:S141-4. [PubMed]
- Helpap B. Morphology and therapeutic strategies for neuroendocrine tumors of the genitourinary tract. Cancer 2002;95:1415-20. [PubMed]
- Cheville JC, Tindall D, Boelter C, et al. Metastatic prostate carcinoma to bone: clinical and pathologic features associated with cancer-specific survival. Cancer 2002;95:1028-36. [PubMed]
- Travis WD. Update on small cell carcinoma and its differentiation from squamous cell carcinoma and other non-small cell carcinomas. Mod Pathol 2012;25 Suppl 1:S18-30. [PubMed]
- Mlika M, Zendah I, Braham E, et al. CD56 antibody: old-fashioned or still trendy in endocrine lung tumors. J Immunoassay Immunochem 2015;36:414-9. [PubMed]
- Mehra R, Tomlins SA, Shen R, et al. Comprehensive assessment of TMPRSS2 and ETS family gene aberrations in clinically localized prostate cancer. Mod Pathol 2007;20:538-44. [PubMed]
- Tomlins SA, Rhodes DR, Perner S, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 2005;310:644-8. [PubMed]
- Bhattacharyya S, Fang F, Tourtellotte W, et al. Egr-1: new conductor for the tissue repair orchestra directs harmony (regeneration) or cacophony (fibrosis). J Pathol 2013;229:286-97. [PubMed]
- Cai C, Wang H, Xu Y, et al. Reactivation of androgen receptor-regulated TMPRSS2:ERG gene expression in castration-resistant prostate cancer. Cancer Res 2009;69:6027-32. [PubMed]
- Mounir Z, Lin F, Lin VG, et al. TMPRSS2:ERG blocks neuroendocrine and luminal cell differentiation to maintain prostate cancer proliferation. Oncogene 2015;34:3815-25. [PubMed]
- Scheble VJ, Braun M, Beroukhim R, et al. ERG rearrangement is specific to prostate cancer and does not occur in any other common tumor. Mod Pathol 2010;23:1061-7. [PubMed]
- Guo CC, Dancer JY, Wang Y, et al. TMPRSS2-ERG gene fusion in small cell carcinoma of the prostate. Hum Pathol 2011;42:11-7. [PubMed]
- Lotan TL, Gupta NS, Wang W, et al. ERG gene rearrangements are common in prostatic small cell carcinomas. Mod Pathol 2011;24:820-8. [PubMed]
- Beltran H, Rickman DS, Park K, et al. Molecular characterization of neuroendocrine prostate cancer and identification of new drug targets. Cancer Discov 2011;1:487-95. [PubMed]
- Glover DM, Leibowitz MH, McLean DA, et al. Mutations in aurora prevent centrosome separation leading to the formation of monopolar spindles. Cell 1995;81:95-105. [PubMed]
- Mehra R, Serebriiskii IG, Burtness B, et al. Aurora kinases in head and neck cancer. Lancet Oncol 2013;14:e425-35. [PubMed]
- Maris JM. Unholy matrimony: Aurora A and N-Myc as malignant partners in neuroblastoma. Cancer Cell 2009;15:5-6. [PubMed]
- Otto T, Horn S, Brockmann M, et al. Stabilization of N-Myc is a critical function of Aurora A in human neuroblastoma. Cancer Cell 2009;15:67-78. [PubMed]
- Mosquera JM, Beltran H, Park K, et al. Concurrent AURKA and MYCN gene amplifications are harbingers of lethal treatment-related neuroendocrine prostate cancer. Neoplasia 2013;15:1-10. [PubMed]
- Lapuk AV, Wu C, Wyatt AW, et al. From sequence to molecular pathology, and a mechanism driving the neuroendocrine phenotype in prostate cancer. J Pathol 2012;227:286-97. [PubMed]
- Chong JA, Tapia-Ramírez J, Kim S, et al. REST: a mammalian silencer protein that restricts sodium channel gene expression to neurons. Cell 1995;80:949-57. [PubMed]
- Schoenherr CJ, Anderson DJ, et al. The neuron-restrictive silencer factor (NRSF): a coordinate repressor of multiple neuron-specific genes. Science 1995;267:1360-3. [PubMed]
- Coulson JM, Edgson JL, Woll PJ, et al. A splice variant of the neuron-restrictive silencer factor repressor is expressed in small cell lung cancer: a potential role in derepression of neuroendocrine genes and a useful clinical marker. Cancer Res 2000;60:1840-4. [PubMed]
- Taylor BS, Schultz N, Hieronymus H, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell 2010;18:11-22. [PubMed]
- Svensson C, Ceder J, Iglesias-Gato D, et al. REST mediates androgen receptor actions on gene repression and predicts early recurrence of prostate cancer. Nucleic Acids Res 2014;42:999-1015. [PubMed]
- Tan HL, Sood A, Rahimi HA, et al. Rb loss is characteristic of prostatic small cell neuroendocrine carcinoma. Clin Cancer Res 2014;20:890-903. [PubMed]
- Sage J. The retinoblastoma tumor suppressor and stem cell biology. Genes Dev 2012;26:1409-20. [PubMed]
- Tzelepi V, Zhang J, Lu JF, et al. Modeling a lethal prostate cancer variant with small-cell carcinoma features. Clin Cancer Res 2012;18:666-77. [PubMed]
- Botta GP, Reichert M, Reginato MJ, et al. ERK2-regulated TIMP1 induces hyperproliferation of K-Ras(G12D)-transformed pancreatic ductal cells. Neoplasia 2013;15:359-72. [PubMed]
- Deng X, He G, Levine A, et al. Adenovirus-mediated expression of TIMP-1 and TIMP-2 in bone inhibits osteolytic degradation by human prostate cancer. Int J Cancer 2008;122:209-18. [PubMed]
- Kim YS, Ahn YH, Song KJ, et al. Overexpression and β-1,6-N-acetylglucosaminylation-initiated aberrant glycosylation of TIMP-1: a "double whammy" strategy in colon cancer progression. J Biol Chem 2012;287:32467-78. [PubMed]
- Gong Y, Chippada-Venkata UD, Galsky MD, et al. Elevated circulating tissue inhibitor of metalloproteinase 1 (TIMP-1) levels are associated with neuroendocrine differentiation in castration resistant prostate cancer. Prostate 2015;75:616-27. [PubMed]
- Simon RA, di Sant'Agnese PA, Huang LS, et al. CD44 expression is a feature of prostatic small cell carcinoma and distinguishes it from its mimickers. Hum Pathol 2009;40:252-8. [PubMed]
- Palapattu GS, Wu C, Silvers CR, et al. Selective expression of CD44, a putative prostate cancer stem cell marker, in neuroendocrine tumor cells of human prostate cancer. Prostate 2009;69:787-98. [PubMed]


