Intraductal carcinoma of prostate (IDC-P): from obscure to significant
The evolution of the concept of IDC-P
Although the concept of “intraductal carcinoma of prostate” (IDC-P) may appear to be a new one, particularly to novices in pathology, it has a longer history than many pathologists realized. Observations of carcinoma cells growing or extending into prostatic ducts and/or acini, either prostatic (acinar) adenocarcinoma, or urothelial carcinoma, or even squamous cell carcinoma, led to the coinage of the term “intraductal carcinoma of the prostate” for describing this phenomenon in literature as early as of 1970 (1-3). Invasion of adjacent benign ducts by prostate adenocarcinoma cells were considered similar to what had been observed in other cancers with mucosal or ductular spreading (such as mammary and urothelial carcinomas), in which the nonneoplastic epithelial cells were replaced by extending neoplastic cells but the ductular structure remained intact (1-3). Several groups, particularly Kovi et al., paid closer attention to the issue of intraductal spreading of prostatic carcinoma. For example, intraductal spreading was reported to be present in 48% of 139 cases of prostate cancer (1). It was also noticed that prostate carcinomas showing cribriform patterns and higher Gleason scores more often contained such intraductal components than non-cribriform cancers (4).
The issue of telling such intraductal spreading from high-grade prostate intraepithelial neoplasia (HGPIN) would naturally occur to anyone practicing diagnostic prostate pathology. Indeed, the concept of HGPIN sometimes (particularly in earlier years of its application) has been used to broadly refer to prostatic intraductal neoplasia, not excluding IDC-P. The reluctance of pathologists to make a diagnosis of carcinoma when ductal structure remained or basal cells were present [either assessed by morphology alone or by immunohistochemistry (IHC) of basal cell markers] may have prevented many pathologists from calling such intraductal lesions as carcinoma (even though they might have believed they were), and chose to fall on the safer side of making a diagnosis of HGPIN (5).
The significance of such intraductal spreading became clearer when more detailed studies came to be available. McNeal and Yemoto (6) found that, out of 130 radical prostatectomy cancer cases, 51 contained intraductal, lumen-spanning or lumen-filling components. In areas away from the invasive cancer, dysplasia/PIN was frequent (1,490 foci) but “intraductal carcinoma” were uncommon (22 foci), lending support to the interpretation that these components were spreading carcinoma rather than in situ lesions or precancers. It was observed that cases with such intraductal components within invasive carcinoma were often associated with large (>0.5 mm) tumor masses in perineural spaces, extensive capsular penetration, and positive surgical margins. Presence of larger amount of such intraductal carcinoma, as well as the amount of grade 4/5 cancer, and the presence of large perineural tumor mass, were also observed to be associated with progression as measured by PSA (6). Wilcox et al. applied the IDC-P criteria proposed by McNeal et al. to differentiate IDC-P from HGPIN. Their study of 252 whole-mount radical prostatectomy specimens again showed association of the presence of IDC-P with higher grade and larger volume of the invasive cancers, more frequent involvement of seminal vesicles, and tumor progression (7).
These studies, together with others, not only led to the gradual clarification of the clinical significance of IDC-P, but also the morphological criteria of its diagnosis, most importantly the requirement of prostate adenocarcinoma cells to fill or at least to span major portions of the ductal lumen, yet with at least partial or focal preservation of the basal cells (6,8,9).
The histological criteria for diagnosis of IDC-P
As IDC-P has been increasingly appreciated over the years, histological diagnostic criteria of IDC-P have been proposed by several groups, focusing either on prostatectomies or needle biopsies. The major features are appreciated by most authors although interobserver differences may still exist in given cases (10).
McNeal and Yemoto described their observations on IDC-P in 1996 (6), based on findings in prostatectomy specimens, particularly those with a volume between 4 and 10 cc of the largest carcinoma (which was found to contain 10% or more of intraductal component). The proposed criteria for this setting included “complete spanning of ductal or acinar lumen by trabeculae of malignant epithelial cells”, or ducts with “higher density” of intraluminal cribriform or solid masses, and that the ducts retained basically normal architecture and mostly intact basal cell layer.
A study by Guo and Epstein published in 2006 (9) addressed the issue of IDC-P on needle biopsies and proposed the criteria of IDC-P for this setting as “malignant epithelial cells filling large acini and prostatic ducts, with preservation of basal cells”, which grew in solid or dense (“where there are more solid than luminal areas”) cribriform pattern; or alternatively, in “loose cribriform or micropapillary pattern” but with either marked atypical nuclei (at least 6-times the size of that in normal epithelium) or non-focal (i.e., more than one ductal) comedonecrosis. It was noted that 88.9% of the cases showed more than one pattern.
Cohen et al. (8) emphasized that the size of the duct space must be at least twice that of benign counterpart, and also proposed some minor criteria such as right angle branching, smooth contours of duct space, and dimorphic cell population, but did not lay stress on the size of nuclei.
Despite some differences in the proposed criteria in different settings, a practical approach emphasizing the major features detailed above, with the most important two (overgrowth of frankly malignant cells within ductal spaces, with at least partially preserved basal cell layer) being required, an overall agreement in diagnosing IDC-P appears to be obtainable in most situations (Figure 1).
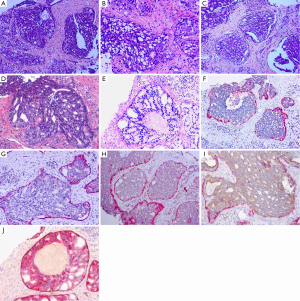
Relationship of IDC-P with invasive carcinoma
Most cases of IDC-P have been reported to be associated with invasive prostate carcinoma, usually of high Gleason score (11). Finding IDC-P without accompanying invasive carcinoma component in needle biopsy is a rare event, and invasive carcinoma of advanced stage and high Gleason score have often been documented on subsequent radical prostatectomy specimens in these cases. In one study, only 27 isolated IDC-P in a series of 45,000 needle biopsies (0.06%) have been identified (9). In another study (12) of 21 cases with only IDC-P in core needle biopsies, analysis of available radical prostatectomy specimens showed pT3a carcinoma in 8 cases (38%), pT3b in 3 (13%), and pT2 in 8 (38%), whereas only 2 (10%) showed no identifiable invasive carcinoma component.
The probability of detecting IDC-P on core needle biopsies varies and has been shown to be associated with concomitant invasive or metastatic carcinoma. In a study of 1,176 biopsy specimens, IDC-P was identified in 10.6% of 312 invasive PCa specimens (13). A study of 901 radical prostatectomies over 7 years identified IDC-P in 155 (17.2%) cases, in comparison with 22 (2.4%) of atypical cribriform lesion (ACL) and 436 (48.4%) of HGPIN (14). In another study of 206 high-risk prostate cancer patients treated with radical prostatectomy, IDC-P component was identified in 104 cases (50%) (15). We recently analyzed advanced stage cases initially diagnosed with metastases (n=278) in our institution (West China Hospital), and identified IDC-P by stringent diagnostic criteria in 57 (20.5%) of the cases (16).
Differential diagnosis
The most important lesion to be differentiated from IDC-P is cribriform HGPIN, since the former represents aggressively spreading carcinoma with poor prognosis, whereas the latter is precancerous. Stringent adherence to the histopathological diagnostic criteria listed above usually will solve the problem in most cases. However, there are situations in which it may be difficult to reach a definitive diagnosis. Recently, the concept of ACL has been proposed in an attempt to resolve difficulties in this regard. ACL has been defined as cytologically malignant cells spanning the longer luminal axes of prostate glands or ducts (solid areas or comedonecrosis were also included), with partial or complete preservation of the basal cell layer (confirmed by immunostains). ACLs are then divided into two categories: cancer-associated ACLs (ACL-PCa) and noncancer-associated ACL (isolated ACL). ACL-PCa is defined as ACLs intermixed with or within 3 mm from the border of infiltrative cancer. And the others are defined as isolated ACL. The authors proposed that ACL-PCa is equivalent to IDC-P, whereas isolated ACL is equivalent to cribriform HGPIN (17,18).
Invasive cribriform prostate carcinoma (Gleason pattern 4, or Gleason 5 if there is comedonecrosis) by definition lacks basal cells, which can be easily identified with IHC. Some histological features, such as branching pattern of duct and lack of glandular confluence, also may aid in the differentiation of IDC-P from infiltrating cribriform carcinoma. The differential diagnosis of IDC-P and invasive cribriform acinar adenocarcinoma may appear to be of less importance clinically since IDC-P is almost always associated with high-grade invasive carcinoma, and both represent aggressive high-grade lesions and both are associated with advanced stage and poor prognosis. However, recent studies indicated that IDC-P associated with invasive high-grade carcinoma might still confer additional prognostic information. For example, we recently analyzed the prognostic significance of IDC-P in advanced stage patients with metastases, and our data indicated that, even in this group of patients, the presence of IDC-P still predicted worse outcome than those without IDC-P (16).
IDC-P should not be confused with ductal adenocarcinoma, although both represent high-grade aggressive forms of prostate adenocarcinoma, often as components of more conventional aggressive acinar adenocarcinoma and may indeed be intermingled with each other. The microscopic morphology of ductal adenocarcinoma is characterized by true papillary (with fibrovascular cores) and/or cribriform growth patterns showing slit-like spaces, and necrosis is common. The cancer cells are often tall columnar and pseudostratified, resembling that of endometrial adenocarcinoma, with large nuclei and nucleoli and high mitotic activity. Although residual basal cells may occasionally be observed in ductal adenocarcinoma, the basal cell layer should be largely absent to warrant unequivocal diagnosis of ductal adenocarcinoma (17).
Another differential diagnosis that must be appreciated when considering IDC-P is the intraductal spread of urothelial carcinoma. Although urothelial carcinoma mostly commonly originate from the urinary bladder, rare occurrence in the prostate proper as a primary also need to be considered. IHC plays important roles in nailing down a diagnosis of urothelial carcinoma in this scenario, which is typically positive for P63, HCK, CK7, and often CK20, but is negative for PSA, thus essentially excluding Pca. A caveat here is the recently described rare P63-positive Pca. The characteristic, so-called “hard”, dense pinkish cytoplasm of urothelial carcinoma with which most uropathologists are familiar also helps in alerting the differentiation.
Differentiation of IDC-P from metastatic or ductal spreading colorectal carcinoma sometimes may be required and may also be facilitated by IHC, as the latter often is characterized by positivity for CK20 and CDX2, and negativity for PSA or PSAP, but careful histological evaluation (for such features as mucinous differentiation) and clinicopathological correlation are also necessary (17).
Molecular studies
Earlier attempts on analysis of loss of heterozygosity (LOH) (including that of TP53 and RB) did not identify similar profiles in IDC-P (LOH common), invasive prostate adenocarcinoma (LOH moderately frequent), and HGPIN (LOH rare) (19,20), which led some authors to propose that IDC-P was distinct from not only HGPIN but also invasive adenocarcinoma. Recent molecular studies, however, have shed more light on this critical issue and demonstrated close relationship of IDC-P and concurrent invasive adenocarcinoma component.
The most interesting molecular evidence perhaps is that of the ERG status, as it has been shown that the majority of IDC-P carried ERG rearrangement, a molecular event also frequently observed in Pca, which involves the ETS transcript factor family genes and can be analyzed by fluorescence in-situ hybridization (FISH). TMPRSS2-ERG gene fusions have been observed in as high as 70% of IDC-P and adjacent invasive prostate carcinoma (17). In one report (21), ERG rearrangement was identified in 75% (36/48) of IDC-P, either by deletion (23/36) or insertion (13/36), but absent (0/16) in isolated ACL (cribriform HGPIN). In addition, the ERG rearrangement status in the IDC-P was consistent with that in adjacent invasive carcinoma when compared (34/34), further supporting that IDC-P is distinct from isolated cribriform HGPIN at the molecular level, and may also help to solve difficult biopsy cases in which IDC-P and HGPIN cannot be ascertained by morphologic criteria (21).
ERG IHC has been used as an alternative means of showing the ERG rearrangement event, and the reported positivity in the literature ranged from 10–40% for Asians and 50–70% for Europeans. However, in our experience at the West China Hospital, ERG positivity appears to be much lower in Chinese patients (107/635, 16.9%), thus may limiting its usefulness (Figure 2). In one study of 31 cases, Schneider and Osunkoya (22) observed all eleven (11/31, 35%) cases showing ERG immunohistochemical positivity in IDC-P were also ERG-positive in the invasive adenocarcinoma component, whereas all the other 20 cases showing ERG negativity in the IDC-P were also ERG-negative in the invasive component. These data, together with the molecular studies mentioned above, all supported the notion that IDC-P shared molecular signatures of the invasive counterpart (22).
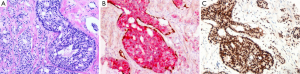
Similarly, PTEN loss has been considered an important molecular event in prostate carcinogenesis and some studies have proposed that PTEN IHC may be useful in distinguishing IDC-P from HGPIN. One study reported that PTEN loss was identified by IHC in 76% (38/50) of IDC-P with concomitant invasive adenocarcinoma, with 58% (29/50) also expressing ERG. Even in biopsies containing isolated intraductal carcinoma, PTEN loss was identified in 61% (20/33), together with 30% (10/33) positivity of ERG. Of the borderline intraductal proliferations, 52% (11/21) showed PTEN loss and 27% (4/15) expressed ERG. These authors observed neither PTEN immunostaining loss nor ERG immunostaining expression in PIN cases (0/19) (23). Another study used a combined immunostain (PTEN, ERG, p63 and CK903) and identified cytoplasmic PTEN loss in 84% (38/45) of the IDC-P and in 100% (15/15) of intraductal cribriform proliferations not fully meeting the criteria of IDC-P, but no PTEN loss was observed in PIN (0/39). At the same time, ERG expression was identified in 58% (26/45) of the intraductal carcinomas and 67% (10/15) of the intraductal cribriform proliferations, but only in 13% (5/39) of PIN. Again, concordance between the PTEN/ERG status of the IDC-P component and the concomitant invasive carcinoma component was high (24). More studies are needed to further evaluate the usefulness of these IHC markers in diagnosing IDC-P.
Other molecular changes in IDC-P are also being investigated. A recent report (25) analyzed the relationship between BRCA2 mutation with IDC-P in men with familial history of PCa by establishing patient-derived xenografts (PDXs) from germline BRCA2 mutation carriers and non-carriers (BRCAX patient) and by performing whole-genome copy number analysis. The authors showed that BRCA2 tumors showed higher incidence of IDC-P in contrast to sporadic Pca, and the genetic profile of IDC-P from matched primary or PDX BRCA2 mutation tumor was similar (25). Whole genome analysis has also been employed to show that the genetic profiles of metastatic subclones share most similarities with concurrent IDC-P, supporting the idea that IDC-P represents more aggressive subclones derived from the invasive component (26).
Clinical significance
IDC-P has been shown to be associated with higher Gleason score, total tumor volume, higher stage or probability of seminal vesicle involvement, early biochemical relapse and tumor progression, as well as to be an independent prognostic factor of progression-free survival (PFS), overall survival (OS), or cancer-specific survival (CSS) (7,14,15,27,28). In addition to this overall impact IDC-P has on prognosis, it has also been proposed that IDC-P sub-patterns defined by the architecture of the central (luminal) cell compartment (A, trabecular; B, cribriform; and C, solid/comedonecrosis) represented progressive dedifferentiation, which might also be correlated with stage, grade, and clinical course (29).
In a recent study we analyzed metastatic prostate cancer patients (n=278) diagnosed by needle biopsy, and identified 57 with IDC-P (57/278, 20.5%), which was found to be a significant independent factor associated with faster occurrence of castration-resistant prostate cancer (CRPC) and poorer OS (16). In another study, we also observed that presence of IDC-P in metastatic CRPC also predicted poorer chemotherapy response (30). It is also interesting to notice that BRCA2 carriers as well as non-carrier patients with IDC-P had significantly worse prognosis compared with BRCA2 carriers and BRCAX patients without IDC-P (25).
Reporting IDC-P
Although the value of reporting the presence of IDC-P in the background of concurrent invasive, high-Gleason grade Pca has been questioned sometimes, given the clinical significance outlined above, particularly the additional adverse prognostic implications it confers (16,30), careful evaluation and report of the presence of IDC-P in either biopsy or radical prostatectomy should probably be recommended.
IDC-P without invasive prostate carcinoma in needle biopsy is rare, and pathologists, especially general pathologists, may feel uncomfortable to make a diagnosis of IDC-P without invasive prostate carcinoma. However, its presence should be communicated in an appropriate way to the urologists to alert that these lesions are usually associated with high-grade and advanced stage prostate cancer, and definitive therapies may be necessary, or at least repeat biopsies should be obtained for further evaluation or to confirm diagnosis of high-grade invasive prostate cancer. It has been recommended that biopsies with a few atypical cribriform glands of “low grade” morphology falling short of definitive IDC-P should be reported as “ACL” (with a comment stating that definitive distinction between cribriform HGPIN and IDC-P cannot be made on the material and a repeat biopsy recommended) (17). Consultation with expert genitourinary pathologists is also recommended when in doubt (11,17).
Acknowledgements
Funding: The authors are supported by grants from the Natural Science Foundation of China (NSFC 81272848, 81272820, 81302225, 81572540).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kovi J, Jackson MA, Heshmat MY. Ductal spread in prostatic carcinoma. Cancer 1985;56:1566-73. [PubMed]
- Catalona WJ, Kadmon D, Martin SA. Surgical considerations in treatment of intraductal carcinoma of the prostate. J Urol 1978;120:259-61. [PubMed]
- Rhamy RK, Buchanan RD, Spalding MJ. Intraductal carcinoma of the prostate gland. J Urol 1973;109:457-60. [PubMed]
- McNeal JE, Reese JH, Redwine EA, et al. Cribriform adenocarcinoma of the prostate. Cancer 1986;58:1714-9. [PubMed]
- Bostwick DG, Brawer MK. Prostatic intra-epithelial neoplasia and early invasion in prostate cancer. Cancer 1987;59:788-94. [PubMed]
- McNeal JE, Yemoto CE. Spread of adenocarcinoma within prostatic ducts and acini. Morphologic and clinical correlations. Am J Surg Pathol 1996;20:802-14. [PubMed]
- Wilcox G, Soh S, Chakraborty S, et al. Patterns of high-grade prostatic intraepithelial neoplasia associated with clinically aggressive prostate cancer. Hum Pathol 1998;29:1119-23. [PubMed]
- Cohen RJ, Wheeler TM, Bonkhoff H, et al. A proposal on the identification, histologic reporting, and implications of intraductal prostatic carcinoma. Arch Pathol Lab Med 2007;131:1103-9. [PubMed]
- Guo CC, Epstein JI. Intraductal carcinoma of the prostate on needle biopsy: Histologic features and clinical significance. Mod Pathol 2006;19:1528-35. [PubMed]
- Iczkowski KA, Egevad L, Ma J, et al. Intraductal carcinoma of the prostate: interobserver reproducibility survey of 39 urologic pathologists. Ann Diagn Pathol 2014;18:333-42. [PubMed]
- Tsuzuki T. Intraductal carcinoma of the prostate: a comprehensive and updated review. Int J Urol 2015;22:140-5. [PubMed]
- Robinson BD, Epstein JI. Intraductal carcinoma of the prostate without invasive carcinoma on needle biopsy: emphasis on radical prostatectomy findings. J Urol 2010;184:1328-33. [PubMed]
- Watts K, Li J, Magi-Galluzzi C, et al. Incidence and clinicopathological characteristics of intraductal carcinoma detected in prostate biopsies: a prospective cohort study. Histopathology 2013;63:574-9. [PubMed]
- Miyai K, Divatia MK, Shen SS, et al. Clinicopathological analysis of intraductal proliferative lesions of prostate: intraductal carcinoma of prostate, high-grade prostatic intraepithelial neoplasia, and atypical cribriform lesion. Hum Pathol 2014;45:1572-81. [PubMed]
- Kimura K, Tsuzuki T, Kato M, et al. Prognostic value of intraductal carcinoma of the prostate in radical prostatectomy specimens. Prostate 2014;74:680-7. [PubMed]
- Zhao T, Liao B, Yao J, et al. Is there any prognostic impact of intraductal carcinoma of prostate in initial diagnosed aggressively metastatic prostate cancer? Prostate 2015;75:225-32. [PubMed]
- Shah RB, Zhou M. Atypical cribriform lesions of the prostate: clinical significance, differential diagnosis and current concept of intraductal carcinoma of the prostate. Adv Anat Pathol 2012;19:270-8. [PubMed]
- Shah RB, Magi-Galluzzi C, Han B, et al. Atypical cribriform lesions of the prostate: relationship to prostatic carcinoma and implication for diagnosis in prostate biopsies. Am J Surg Pathol 2010;34:470-7. [PubMed]
- Dawkins HJ, Sellner LN, Turbett GR, et al. Distinction between intraductal carcinoma of the prostate (IDC-P), high-grade dysplasia (PIN), and invasive prostatic adenocarcinoma, using molecular markers of cancer progression. Prostate 2000;44:265-70. [PubMed]
- Bettendorf O, Schmidt H, Staebler A, et al. Chromosomal imbalances, loss of heterozygosity, and immunohistochemical expression of TP53, RB1, and PTEN in intraductal cancer, intraepithelial neoplasia, and invasive adenocarcinoma of the prostate. Genes Chromosomes Cancer 2008;47:565-72. [PubMed]
- Han B, Suleman K, Wang L, et al. ETS gene aberrations in atypical cribriform lesions of the prostate: Implications for the distinction between intraductal carcinoma of the prostate and cribriform high-grade prostatic intraepithelial neoplasia. Am J Surg Pathol 2010;34:478-85. [PubMed]
- Schneider TM, Osunkoya AO. ERG expression in intraductal carcinoma of the prostate: comparison with adjacent invasive prostatic adenocarcinoma. Mod Pathol 2014;27:1174-8. [PubMed]
- Morais CL, Han JS, Gordetsky J, et al. Utility of PTEN and ERG immunostaining for distinguishing high-grade PIN from intraductal carcinoma of the prostate on needle biopsy. Am J Surg Pathol 2015;39:169-78. [PubMed]
- Lotan TL, Gumuskaya B, Rahimi H, et al. Cytoplasmic PTEN protein loss distinguishes intraductal carcinoma of the prostate from high-grade prostatic intraepithelial neoplasia. Mod Pathol 2013;26:587-603. [PubMed]
- Risbridger GP, Taylor RA, Clouston D, et al. Patient-derived xenografts reveal that intraductal carcinoma of the prostate is a prominent pathology in BRCA2 mutation carriers with prostate cancer and correlates with poor prognosis. Eur Urol 2015;67:496-503. [PubMed]
- Lindberg J, Kristiansen A, Wiklund P, et al. Tracking the origin of metastatic prostate cancer. Eur Urol 2015;67:819-22. [PubMed]
- Trudel D, Downes MR, Sykes J, et al. Prognostic impact of intraductal carcinoma and large cribriform carcinoma architecture after prostatectomy in a contemporary cohort. Eur J Cancer 2014;50:1610-6. [PubMed]
- Van der Kwast T, Al Daoud N, Collette L, et al. Biopsy diagnosis of intraductal carcinoma is prognostic in intermediate and high risk prostate cancer patients treated by radiotherapy. Eur J Cancer 2012;48:1318-25. [PubMed]
- Cohen RJ, McNeal JE, Baillie T. Patterns of differentiation and proliferation in intraductal carcinoma of the prostate: significance for cancer progression. Prostate 2000;43:11-9. [PubMed]
- Chen Z, Chen N, Shen P, et al. The presence and clinical implication of intraductal carcinoma of prostate in metastatic castration resistant prostate cancer. Prostate 2015;75:1247-54. [PubMed]


