Pathologic features and clinical outcome of central neurocytoma: analysis of 15 cases
Objective: To get better recognition of central neurocytoma and diminish misdiagnosis.
Methods: A retrospective review identified 15 cases of central neurocytoma. All cases of central
neurocytoma were analyzed for their clinical symptoms, pathologic changes, immunohistochemical staining,
prognosis and differential diagnosis. Clinical follow up was performed.
Results: There were 8 males and 7 females aged 10-64 years (median 32.93 years). The most common
presenting symptoms were those related to increased intracranial pressure (ICP), including headache (100%),
papilledema (93%) and vomiting (80%). All tumors were located in the ventricular system. The tumors were
composed of uniform cells with round nuclei and a fine chromatin pattern, and in some areas, small cells with
perinuclear halo could be seen. In particular, the anuclear areas may have a fine fibrillary matrix (neuropil).
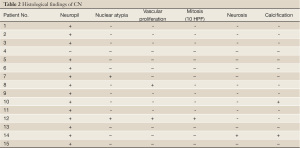
Nuclear atypia and vascular proliferation appeared in two cases, respectively. Focal necrosis could be seen
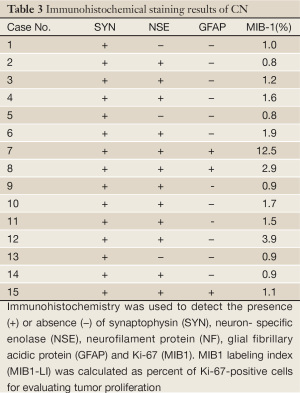
in one case. Immunohistochemical findings included expression of synaptophysin (15/15), neuron specific
enolase (12/15) and glial fibrillary acidic protein (GFAP) (3/15). MIB-1 proliferation index ranged from 0.8-
12.5%, and was more than 2% in 3 of 15 cases assessed. Follow-up information of 11 patients was available.
Conclusions: Central neurocytoma has a favorable prognosis in general, but in some cases, the clinical
course could be aggressive. Increase of GFAP positivity, proliferation index and vascular proliferation might
suggest a more malignant process.
Key words: Central neurocytoma; histopathology; immunohistochemistry; proliferation; MIB-1 labeling index
Introduction
Centraleurocytoma (CN) is a kind of uncommon tumor arising in the central nervous system, most descriptions of which available in literature are in the form of isolated case reports and small series. CN approximately accounts for 0.25-0.50% of all primary central nervous system tumors (2). This kind of tumor is composed of uniform round cells that show evidence of neuronal differentiation (1,2), and typically occurs in the supratentorial ventricular system in young adults. Owing to this rare incidence, diagnosis and management of this neoplasm remain controversial. Although the clinical course is usually benign, cases with poor outcome have been reported (3). It has not yet been clearly established which features of CN are of prognostic significance. In this study, the relationship among the histological characteristics, proliferation potential and clinical process of CN was examined.
Materials and methods
The specimens of 15 CN patients were retrieved from Department of Pathology in Chongqing Medical University. All cases had been diagnosed between 1988 and 2008. The diagnosis of CN was based on a combination of clinical information, morphologic examination and immunohistochemical results, and was confirmed by two pathologists. The specimens were fixed in 10% formalin and embedded in paraffin. Paraffin-embedded sections (4 µm) were obtained and hematoxylin and eosin (H&E) staining was performed. All histological slides from each case were examined for the presence of atypical features, including cellular atypia, MIB-1 proliferation index, vascular endothelial proliferation and necrosis. Immunohistochemical staining was performed on formalin-fixed, paraffin-embedded tissue sections of the 15 cases. Microsections were deparaffinized and subjected to antigen retrieval by steaming (20 min at 80 ℃). Then, the slides were incubated at room temperature together with antibodies. Antibodies were detected using the streptavidin-perosidase (S-P) method, with diaminobenzidine as the chromogen. The proliferation potential was assessed on MIB-1 immunostained sections (Ki-67, Gene Co., Ltd.). A minimum of 1,000 cells were counted in the area of highest proliferative activity, and the number of labeled cells was expressed as a percentage of the total number of cells [MIB-1 labeling index (LI)]. The MIB-1 LI was considered elevated when it was greater than 2% (4).
Results
Clinical features
The main clinical features of the 15 patients with CN are shown in Table 1. There were 8 males and 7 females aged 10-64 years (median 32.93 years). The most common presenting symptoms were those related to increased intracranial pressure (ICP), including headache (15/15, 100%), papilledema (14/15, 93%), and vomiting (12/15, 80%). Memory disturbance was presented in 1 patient. Dementia was presented in 1 patient. Computer tomography (CT) and magnetic resonance imaging (MRI) scans of the 15 patients showed that all tumors were located in the ventricular system, 7 were in septum pellucidum, 5 were in the left or right ventricles and the other 3 were in the third ventricle. The largest tumor was 6.5 cm × 6.0 cm × 4.0 cm, and the smallest one was 3.0 cm × 2.5 cm × 2.0 cm.

Full table
Pathological findings
CN is generally grayish in color, resembling the grey matter, well-circumscribed, with areas of hemorrhage. Grittiness experienced while dissecting resected specimen is due to the presence of calcification.
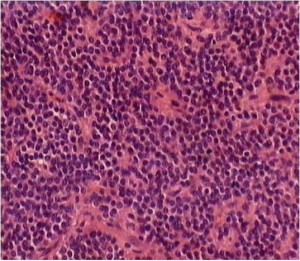
The tumor samples of all cases were stained with H&E for routine histopathologic processing. Under microscope, CN was well-differentiated tumor with benign histological feature, which may display varied histological architecture even in the same specimen. H&E stained sections often revealed that the tumor is composed of uniform, small-to-medium-sized cells with rounded nuclei, finely stippled chromatin and inconspicuous nucleoli, along with scant cytoplasm. The histopathologic appearance of CN may be similar to that of oligodendroglioma. This was particularly true for frozen section preparations. Both neoplasms had small uniform cells with rounded nuclei and scant cytoplasm resembling perinuclear halos (‘fried egg’ appearance). The tumor cells were dense in some areas and alternate with anuclear, less dense tumor parts. In particular, the anuclear areas may have a fine fibrillary matrix (neuropil). Vascularity was represented by long, thin-walled capillary-sized vessels, which were arranged in a linear arborizing pattern, imparting an endocrine appearance (Figure 1). In many cases, thin-walled dilated vascular channels, and foci of calcification were readily identified. Cases 7 and 12 showed nuclear atypia. Case 12 had a significantly higher mitotic rate than the others in this series. Vascular proliferation could be seen in cases 8 and 12. Case 14 showed focal necrosis. Calcification could be observed to a limited extent in cases 10 and 14 (Table 2).
Full table
Evaluation of immunohistochemical staining
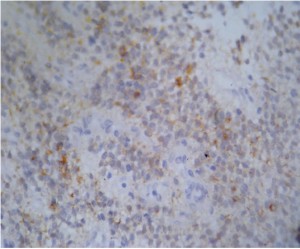
Immunohistochemical staining results for each case are listed in Table 3. Diffuse and strong synaptophysin immunoreactivity was seen in all cases (15/15) (Figure 2). Neuron specific enolase (NSE) immunostaining was positive in four-fifth of cases (12/15). Glial fibrillary acidic protein (GFAP) immunostaining was positive in one-fifth of cases (3/15). The MIB-1 proliferation index ranged from 0.8-12.5%, and was more than 2% in 3 of 15 cases assessed (Table 3).
Full table
Follow-up
Follow-up information was available for 11 patients. The follow-up period ranged from 0.5 to 10.5 years. Six patients with total tumor removal have no evidence of recurrence. In the three patients with subtotal removal and postoperative radiation, recurrence or progression of tumor had also not yet been found. Case 7 originally did favorably after partial resection of the tumor. Sixteen months later, however, he died of extensive hemorrhage, associated with the residual tumor which had progressed since surgery. Case 12 remained clinically well at the half-year follow up. The CT scan, however, showed definite progression of the tumor. Four patients were lost in the follow-up period.
Discussion
Clinical features
CN was first described in 1982 by Hassoun et al. (2). It is a well-differentiated intraventricular tumor with characteristic of neuronal origin and a relatively benign clinical course. The true incidence of the tumor, however, remains unknown and is estimated ranging from 0.1% to 0.5% of all the primary central nervous system tumors. The patients with CN are aged from 5 to 76 years (mean 34 years). There is a peak during the second and third decades of life, but patients older than 60 years have been reported. Both sexes are equally affected (5). Most neurocytomas are midline supratentorial lesions straddling the lateral and third ventricles, often in relation to the foramen of Monro and arising from the septum pellucidum, fornix, or walls of the lateral ventricles. But neurocytomas in the cerebrum, cerebellum, brain stem and spinal cord, which were called extraventricle (parenehymal) neurocytic neoplasms, had been found (6).
Here we reported 15 cases of CN. The mean age of the patients is 32.93 years. There are 8 males and 7 females. The most common presenting symptoms were related to increased ICP, including headache, papilledema and vomiting. All tumors were located in the ventricular system: 7 in septum pellucidum, 5 in the bilateral ventricles and the other 3 in the third ventricle. These tumors generally came to clinical attention because of the mass effect produced by the tumor and secondary hydrocephalus. The patients complained of headache, nausea, vomiting, and memory and visual disturbances. CT and MRI results revealed that the tumors in the ventricle system were usually a big mass.
Macro- and microscopic characteristics
Macroscopically, neurocytoma occurs as a soft, ovoid, lobulated to nodular mass that is generally well circumscribed. The neoplasm is generally grayish in color, resembling the grey matter, with areas of hemorrhage. Grittiness experienced while dissecting resected specimen is due to the presence of calcification (5).
In the 15 cases of our series, all tumors were located in the ventricular system. The largest tumor was 6.5 cm × 6.0 cm × 4.0 cm and the smallest one was 3.0 cm × 2.5 cm × 2.0 cm. These neurocytomas are generally grayish in color, with areas of hemorrhage. Calcification was found in 2 of 15 cases.
Microscopically, neurocytomas are well-differentiated tumors with benign histological feature, which may display varied histological architecture even in the same specimen. These tumors are composed of uniform, small-to-medium-sized cells with rounded nuclei, finely stippled chromatin (“salt and pepper” chromatin) and inconspicuous nucleoli, along with scant eosinophilic cytoplasm and in some areas have small cells with perinuclear halos, which resemble oligodendroglioma. Cells are usually closely apposed but may also be set within a background of finely filamentous stroma having a neuropil-like quality. In places, tumors display dense cellular areas alternating with fibrillary/acellular areas. The latter components are mainly perivascular and have a fine fibrillary neuropil matrix mimicking “rosettes of ependymoma”. In many cases, thin-walled dilated vascular channels, and foci of calcification are readily identified (5).
Histopathologic changes of our groups were similar to literature. The tumor was composed of uniform small round cells with rounded nuclei surrounded by prominent perinuclear halos, contained clear or slightly eosinophilic cytoplasm, and had nuclus-free areas of neuropil, a fine, interlaced vascular pattern.
Immunohistochemistry
Strong immunostaining for synaptophysin has been recognized as the most suitable and reliable diagnostic marker. Typically, synaptophysin immunoreactivity is noted in the neuropil, especially in fibrillary zones and perivascular cell-free areas, and not in the cell bodies of normal neurons. Tumor cells have been reported to express neuron-specific enolase. Immunostaining for synaptophysin (SYN) and neuron- specific enolase (NSE) confirm the neuronal nature of the neoplasm. Neuronal nuclear antigen (NeuN) expression is generally associated with tumor cells displaying terminal neuronal differentiation and is often helpful in resolving ambiguous synaptophysin staining (6). The significance of GFAP reactivity in tumor cells is difficult to explain, but in vitro experiments with neurocytomas have shown a shift from synaptophysin to GFAP expression with cell passages (6). It has been suggested that CN originates from bipotential (neuronal and astrocytic) progenitor cells in the periventricular region that persist into adulthood (7). In our cases, immunostaining studies suggested bipotential differentiation of the neoplasm, and the expressive rates of SYN, NSE and GFAP were 15/15, 12/15 and 3/15, respectively.
Biological behavior and prognosis
Although anaplasia has been demonstrated in CN, the influence of this feature on prognosis is uncertain (7). Yasargil et al. (8) reported that 2 of the 8 patients had evidence of anaplasia and were treated with post-operative radiation after total excision. Those tumors did not relapse at the time of the report after a follow-up of 5 and 12 months. Three patients in the same study had recurrences 38-92 months after total excision and none had evidence of anaplasia. Thus, it is not clear if tumors with anaplasia have a higher relapse rate or if they need additional treatment. Yet it appears that some cases of CN may be more aggressive despite benign histological feature (8).
Most reports indicated that CN has limited growth potential (4). In one study of multiple proliferation markers [including silver nucleolar organizer region (AgNOR) technique, proliferating cell nuclear antigen (PCNA), and MIB-1 LI], only MIB-1 index tended to be higher in CN with mitosis and necrosis (9). In a larger study with 36 cases of CN, the MIB-1 LI showed that a LI of 2% might be critical in determining recurrence (4). The patients with an MIB-1 LI <2% had a 22% relapse rate compared to 63% when the MIB-1 LI was over 2% for the observation period of 150 months (4). Our results are in accord with these reports.
Molecular genetics
It appears that CN is genetically distinct from neuroblastoma and oligodendroglioma. Twenty-six percent of neuroblastoma has N-myc amplification and/or 1p36 loss of heterozygosity (LOH). These abnormalities are rare in CN (10). In addition, in oligodendroglioma, LOH of 1p and 19q are important aberrations. Tong et al. (10) found LOH of 1p in 6/9 and LOH of 19q in 5/9 CN. In Fujisawa et al. (11), all 8 cases of CN did not have allelic loss on 1p and 19q. Thus, it is unclear if these genetic markers are relevant for CN. Mutations of p53 tumor suppressor gene have also not been detected in CN (11).
Histological origin
Currently, it remains controversial as to whether neurocytomas arise from cells committed to neuronal phenotype (12) or from bipotential cells in the periventricular matrix (13). In vitro cultures by Westphal et al. (14) and others (15-17) have revealed that CN have properties reminiscent of bipotential precursor cells, which can exhibit both glial and neuronal differentiation. This bipotential property possibly explains the GFAP immunopositive cell population observed in CN. Our immunostaining results showed that the expressive rates of SYN, NSE and GFAP were 15/15, 12/15 and 3/15 respectively, which supported the notion of bipotential differentiation.
Differential diagnosis
In this group of CN we presented, they had been initially diagnosed as other tumors based on light microscopy, including four cases of oligodendroglioma, and two cases of ependymoma. Despite the aforementioned characteristic histological appearances, there are a wide variety of differential diagnoses of CN (18-22): (I) Oligodendroglioma. Owing to their cytoplasmic vacuolations and monomorphic histologic appearances, neurocytomas need to be differentiated from oligodendroglial tumors. Awareness of the condition, an intraventricular localization, along with histological features of cellular uniformity, neuropil islands, salt and pepper chromatin, eosinophilic granular bodies, and lack of infiltrated brain parenchyma, suggest a diagnosis of neurocytoma (6). Immunoreactivity for synaptophysin in neurocytomas substantiates histology. (II) Ependymoma. An intraventricular tumor with solid, noninfiltrating pattern along with perivascular pseudorosettes warrants a differential diagnosis of ependymoma. However, characteristic features of nuclear roundness with speckled chromatin and comparatively ill-defined perivascular rosettes suggest neurocytic origin, which are confirmed by synaptophysin immunopositivity of rosettes along with GFAP immunonegativity (5).
Acknowledgements
This work was supported by the Natural Science Foundation of Chongqing Science and Technology Committee (CSTC, 2006BB5298) and Sci & Tech Project of Chongqing Municipal Education Commission (KJ080302).
Disclosure: The authors declare no conflict of interest.
References
- Choudhari KA, Kaliaperumal C, Jain A, et al. Central neurocytoma: a multi-disciplinary review. Br J Neurosurg 2009;23:585-95.
- Hassoun J, Gambarelli D, Grisoli F, et al. Central neurocytoma. An electron-microscopic study of two cases. Acta Neuropathol 1982;56:151-6.
- Mrak RE. Malignant neurocytic tumor. Hum Pathol 1994;25:747-52.
- Söylemezoglu F, Scheithauer BW, Esteve J, et al. Atypical central neurocytoma. J Neuropathol Exp Neurol 1997;56:551-6.
- Sharma MC, Deb P, Sharma S, et al. Neurocytoma: a comprehensive review. Neurosurg Rev 2006;29:270-85; discussion 285.
- Brat DJ, Scheithauer BW, Eberhart CG, et al. Extraventricular neurocytomas: pathologic features and clinical outcome. Am J Surg Pathol 2001;25:1252-60.
- Vasiljevic A, François P, Loundou A, et al. Prognostic factors in central neurocytomas: a multicenter study of 71 cases. Am J Surg Pathol 2012;36:220-7.
- Chen CM, Chen KH, Jung SM, et al. Central neurocytoma: 9 case series and review. Surg Neurol 2008;70:204-9.
- Chen CL, Shen CC, Wang J, et al. Central neurocytoma: a clinical, radiological and pathological study of nine cases. Clin Neurol Neurosurg 2008;110:129-36.
- Rodriguez FJ, Mota RA, Scheithauer BW, et al. Interphase cytogenetics for 1p19q and t(1;19)(q10;p10) may distinguish prognostically relevant subgroups in extraventricular neurocytoma. Brain Pathol 2009;19:623-9.
- Fujisawa H, Marukawa K, Hasegawa M, et al. Genetic differences between neurocytoma and dysembryoplastic neuroepithelial tumor and oligodendroglial tumors. J Neurosurg 2002;97:1350-5.
- Figarella-Branger D, Pellissier JF, Daumas-Duport C, et al. Central neurocytomas. Critical evaluation of a small-cell neuronal tumor. Am J Surg Pathol 1992;16:97-109.
- Vallat-Decouvelaere AV, Gauchez P, Varlet P, et al. So-called malignant and extra-ventricular neurocytomas: reality or wrong diagnosis? A critical review about two overdiagnosed cases. J Neurooncol 2000;48:161-72.
- Westphal M, Stavrou D, Nausch H, et al. Human neurocytoma cells in culture show characteristics of astroglial differentiation. J Neurosci Res 1994;38:698-704.
- Ishiuchi S, Nakazato Y, Iino M, et al. In vitro neuronal and glial production and differentiation of human central neurocytoma cells. J Neurosci Res 1998;51:526-35.
- Sharma MC, Rathore A, Karak AK, et al. A study of proliferative markers in central neurocytoma. Pathology 1998;30:355-9.
- Yano H, Ohe N, Shinoda J, et al. Immunohistochemical study concerning the origin of neurocytoma--a case report. Pathol Oncol Res 2009;15:301-5.
- Feiden S, Feiden W. WHO classification of tumours of the CNS: revised edition of 2007 with critical comments on the typing und grading of common-type diffuse gliomas. Pathologe 2008;29:411-21.
- Chen CM, Chen KH, Jung SM, et al. Central neurocytoma: 9 case series and review. Surg Neurol 2008;70:204-9.
- Bazarbacha HM, Zouaoui W, Sebai R, et al. Central neurocytoma. Tunis Med 2006;84:572-7.
- Rajesh LS, Jain D, Radotra BD, et al. Central neurocytoma: a clinico-pathological study of eight cases. Indian J Pathol Microbiol 2006;49:543-5.
- De Tommasi A, D’Urso PI, De Tommasi C, et al. Central neurocytoma: two case reports and review of the literature. Neurosurg Rev 2006;29:339-47.