Aberration of the Wnt signaling pathway in pulmonary fatal adenocarcinoma: a case report
Introduction
Well-differentiated fetal adenocarcinoma, known as low grade adenocarcinoma of the fetal lung type, is a rare pulmonary neoplasm that is originally recognized as a variant of pulmonary blastoma that lacks sarcomatous features. The updated World Health Organization (WHO) classification lists four rare variants of invasive adenocarcinoma of the lung: invasive mucinous adenocarcinoma (formerly known as mucinous bronchioloalveolar carcinoma), colloid adenocarcinoma, fetal adenocarcinoma, and enteric adenocarcinoma.
β-catenin plays a dual role in cells as a major structural component of the cadherin-catenin complex and as a pivotal signaling molecule in the Wnt signaling pathway. Wnt signaling cascades can be divided into a canonical or Wnt/β-catenin dependent pathway and the non-canonical or β-catenin-independent pathway. In the presence of Wnt stimulation (on-state), it allows stabilization of β-catenin, resulting in the accumulation of free cytosolic β-catenin. The elevated β-catenin can translocate to the nucleus where it forms a complex with T cell factor/lymphoid enhancer binding factor (TCF/LEF) to stimulate the expressions of Wnt-target molecules, like c-Myc, Cyclin D1 and MMP7 (1,2).
In this study, we described the pathologic features of well-differentiated fetal adenocarcinoma and centered on the immunohistochemical expressions of Wnt1, β-catenin, c-Myc, Cyclin D1 and MMP7 to evaluate the regulation mechanism of β-catenin.
Case description
Clinical history
A 58-year-old woman was referred to Jilin Cancer Hospital, after a chance of detection on chest X-ray of a well-defined mass in the right upper lobe. A computed tomography (CT) scan of the chest was performed, which demonstrated a homogeneous well-defined perihilar mass without calcifications located in the right upper lobe and fully surrounded by aerated parenchyma. No hilar or mediastinal node enlargement or additional parenchymal lesions were detected. At the time of hospitalization, the results of the physical examination of the patient were completely normal. A right upper lobectomy with mediastinal lymphnode sampling was performed on March 11, 2013.
Pathologic features
Gross examination revealed a single, 5.5 cm × 5.0 cm × 5.0 cm, firm-tan-white mass without pleural involvement. At the light microscopy, the tumor revealed sharp borders fully surrounded by normal lung tissue. The tumor was composed of well differentiated glands lined by a single row of columnar cells, with occasionally subnuclear vacuolization, resembling endometrial glands (Figure 1). The definitive diagnosis of well-differentiated fetal adenocarcinoma of the lung was made, and pathologic staging was T2bN0M0 (Stage IIa).
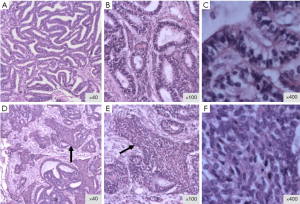
Immunohistochemistry
The immunohistochemistry protocol was as follows. Four-micrometer sections were deparaffinized in xylene and rehydrated in graded ethanol. The succeeding steps were performed automatically at 37 °C using the Benchmark® XT Slide Staining System Specifications (Ventana Medical Systems, Tucson, AZ, USA). Endogenous peroxidases were quenched with 1% H2O2 for 4 min. The sections were incubated with the rabbit polyclonal antibody for Wnt1 (H-89, Santa Cruz, dilution 1:100), the mouse monoclonal antibody for β-catenin (5H10, Zymed Laboratorys, dilution 1:1,000), the mouse monoclonal antibody for c-Myc (9E11, Santa Cruz, dilution 1:5,000), the rabbit monoclonal antibody for Cyclin D1 (SP4, Thermo Fisher Scientific, dilution 1:100) and the mouse monoclonal antibody for MMP7 (JL07, Santa Cruz, dilution 1:100). The secondary biotinylated antibody was incubated for 8 min. The slides were stained using a diaminobenzidine (DAB) detection kit and counterstained with haematoxylin.
Immunohistochemistry showed two different patterns between glandular structure and budding solid nest. The glandular structures showed TTF-1 (+), CK7 (+), P63 (–), CK5/6 (–), CK20 (–), CDX-2 (–), CD56 (–), Synaptophysin (–), EGFR (+), Ki-67 (+/30%). However, the solid nests showed TTF-1 (–), CK7 (–), P63 (–), CK5/6 (–), CK20 (–), CDX-2 (–), CD56 (+), synaptophysin (+), S-100 (+), Ki-67 (+/5%) (Figure 2).
For the expressions of Wnt signaling pathway molecules, the tumor cells showed elevated nuclear/cytoplasmic expression of β-catenin, particularly within the solid nests. Moreover, positive expressions of Wnt1, c-Myc, Cyclin D1 and MMP7 were also especially prominent in solid nests, but not in glandular structure, which accompanied aberrant expression of β-catenin (Figure 3).
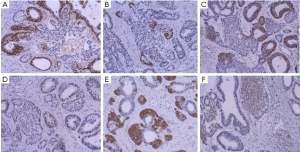
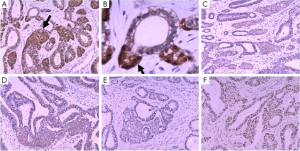
Discussion
Fetal adenocarcinoma is a rare type of lung cancer with an incidence less than 0.1%, first described by Kradin et al. (3) as one subtype of pulmonary blastoma resembling the epithelial component of the fetal lung without a sarcomatous component. In 1991, Koss et al. (4) reported 28 cases consisted of 13 men and 15 women with the mean age 33 years old, and the 5-year survival rate was 81%. We also summarized and reviewed the cases reported in China from 2000 to 2013. In series we reviewed, the patients with ages from 6 to 48 years, and the tumor diameters ranged from 1.2 to 7 cm. Most of reported cases were coded as pathologic stage I, and the 5-year survival mortality rate of 15% to 20%.
We demonstrated that immunohistochemistry showed two different patterns between glandular structures and small solid nests. The solid epithelial cells were immunohistochemically positive for several neuroendocrine markers, such as CD56, synaptophysin and S-100, and entirely negative for TTF-1, CK7, P63, CK5/6, CK20, CDX-2. In contrast, glandular structure cells were negative for neuroendocrine markers. Previous studies also emphasized that fetal adenocarcinoma expressed neuroendocrine markers, but few studies verified two different patterns between glandular structures and small solid nests (5-8).
In this study, we found that Wnt signaling molecules also showed two different patterns between glandular structures and small solid nests like neuroendocrine markers. In the solid nests, elevated nuclear/cytoplasmic localization of β-catenin accompanied Wnt1 overexpression, positive expressions of Wnt target genes like c-Myc, Cyclin D1and MMP7. It demonstrated that the alteration of β-catenin might be regulated by the Wnt signaling pathway, but not cadherin-catenin system in fatal adenocarcinoma. Nakatani et al. (8,9) have reported elevated nuclear/cytoplasmic localization of β-catenin in fetal adenocarcinoma, but not in conventional pulmonary adenocarcinomas, suggesting a potential diagnostic role for β-catenin immunohistochemistry in the differential diagnosis of fetal adenocarcinoma. Sheehan et al. (10) also demonstrated the diagnostic utility of β-catenin immunohistochemistry in the differential diagnosis of fetal adenocarcinoma. To the best of our knowledge, the regulation mechanism of β-catenin in fatal adenocarcinoma is first reported in the present case.
In summary, elevated nuclear/cytoplasmic localization of β-catenin accompanied Wnt1 overexpression, upregulation of Wnt target genes like c-Myc, Cyclin D1and MMP7 in fatal adenocarcinoma. It demonstrated that the altertation of β-catenin might be regulated by the Wnt signaling pathway, but not cadherin-catenin system in fatal adenocarcinoma.
Acknowledgements
This work was supported by the grant of Department of Health, Jilin province, Republic of China (2013Q018 to Xu X).
Disclosure: The authors declare no conflict of interest.
References
- Barker N, Clevers H. Catenins, wnt signaling and cancer. Bioessays 2000;22:961-5. [PubMed]
- You Z, Saims D, Chen S, et al. Wnt signaling promotes oncogenic transformation by inhibiting c-myc-induced apoptosis. J Cell Biol 2002;157:429-40. [PubMed]
- Kradin RL, Young RH, Dickersin GR, et al. Pulmonary blastoma with argyrophil cells and lacking sarcomatous features (pulmonary endodermal tumor resembling fetal lung). Am J Surg Pathol 1982;6:165-72. [PubMed]
- Koss MN, Hochholzer L, O’Leary T. Pulmonary blastomas. Cancer 1991;67:2368-81. [PubMed]
- Yu L, Li X, Yang W. Pulmonary blastoma metastatic to the ovary. Int J Gynecol Pathol 2009;28:59-62. [PubMed]
- Huysentruyt CJ, Vandevijver NM, Jan van Suylen R, et al. Adenocarcinoma of the fetal lung-type metastatic to the ovary. Int J Gynecol Pathol 2010;29:339-40. [PubMed]
- Luo CQ, Yeung SC, Liu ZG, et al. Pulmonary well-differentiated fetal adenocarcinoma with platelet-derived growth factor receptor (PDGFR)α expression. Cancer Biol Ther 2012;13:1384-9. [PubMed]
- Nakatani Y, Kitamura H, Inayama Y, et al. Pulmonary adenocarcinomas of the fetal lung type: a clinicopathologic study indicating differences in histology, epidemiology, and natural history of low-grade and high-grade forms. Am J Surg Pathol 1998;22:399-411. [PubMed]
- Nakatani Y, Masudo K, Miyagi Y, et al. Aberrant nuclear localization and gene mutation of beta-catenin in low-grade adenocarcinoma of fetal lung type: up-regulation of the wnt signaling pathway may be a common denominator for the development of tumors that form morules. Mod Pathol 2002;15:617-24. [PubMed]
- Sheehan KM, Curran J, Kay EW, et al. Well differentiated fetal adenocarcinoma of the lung in a 29 year old woman. J Clin Pathol 2003;56:478-9. [PubMed]


