“Wrapping the gastroduodenal artery stump” during pancreatoduodenectomy reduced the stump hemorrhage incidence after operation
Introduction
Pancreaticoduodenectomy (PD) is a standard surgical procedure for periampullary tumors with very high morbidity. The gastroduodenal artery stump (GDAS) hemorrhage is one of the potentially fatal complications after PD, often occurs 1 to 4 weeks (1-13). GDAS bleeding is usually considered to be correlated with local inflammation and corrosion due to pancreatic leakage (2-4,7-13). Although, as the octreotide and somatostatin widely used and the pancreaticojejunostomy methods gradually improved, the overall incidence of pancreatic fistula (PF) decreased, but now it is still about 2-22% (1-19). PF is difficult to avoid completely (20), thus the risk of GDAS corroded by pancreatic juice is hard to avoid completely.
Maeda et al. reported omental flap could be used to cover the vessels during PD, and it was benefit to reduce postoperative intra-abdominal bleeding and infection (21). Sakamoto et al. indicated wrapping the GDAS using the falciform ligament during PD is useful for protecting the stump of the gastroduodenal artery from pancreatic juice and for preventing hemorrhages (22). But, recently a new retrospective study revealed that the use of omentum or falciform ligament did not decrease complications after PD (23).
We designed a retrospective historical cohort study to investigate whether wrapping the GDAS using the teres hepatis ligamentum during PD could decrease the rate of GDAS hemorrhage.
Patients and methods
Group assignment
We retrospectively reviewed complications of 280 patients (175 males and 105 females; age 12 to 76 years, average 55.7±10.4 years) who accepted PD for malignant (n=269) and benign (n=11) diseases in the Biliary Tract One Department of Eastern Hepatobiliary Surgery Hospital from January 2005 to December 2012. According to whether wrapping the stump of gastroduodenal artery, patients were divided into two groups. A total of 140 consecutive patients (85 males and 55 females; average 55.8±10.0 years) accepted the “wrapping” procedure during PD (wrapping group); the other 140 consecutive not wrapping patients (85 males and 55 females; average 55.7±10.8 years) were selected as controls (non-wrapping group). Age, sex, preoperative data, estimated intraoperative blood loss, postoperative complications, pathologic parameters and hospitalization time were compared between two groups.
Surgical approach
All of 280 patients underwent the conventional pancreaticojejunostomy. PD extent: distal-end stomach (more than 50% of whole stomach), duodenum, pancreatic head and uncinate process of pancreas, gallbladder and common bile duct were resected. Lymph node dissection extent: routine dissection at number 3, 4, 5, 6, 8, 9, 12 13 series of lymph nodes were performed. GDAS treatment: stitch ligature with 1-0 silk suture with needle, followed by number 4 silk suture ligation to strengthen was conducted. GDAS was exposed in non-wrapping group, but wrapped in wrapping group. Pancreatic-enteric anastomosis: end-to-side reconstruction, rather than duct-to-mucosa was performed. Silica gel tube was detained in the pancreatic duct to drain the pancreatic juice. Hepatic duct jejunum anastomosis: end-to-side anastomosis between common hepatic duct stump and jejunum side wall was performed and T tube was detained to drain the bile. Gastroenteric anastomosis: anastomosis between posterior wall of remnant stomach and side wall of jejunum was performed. The interval between gastrointestinal stomas and chol-intestinal stomas was about 40 cm. Jejunum side to side anastomosis (Braun anastomosis): side-to-side anastomosis was performed between input and output jejunums at about 10 cm away from gastrointestinal stomas. Peritoneal cavity drainage tube placement: one drainage tube was put in front of pancreas-intestinal stomas and another one was put behind chol-intestinal stomas.
Postoperative treatment
All patients received intensive care for at least 12 hours in ICU wards. Somatostatin was infused into patients at 4 mL/h (6 mg dissolved in 100 mL physiological saline) by minipump or 0.1 mg octreotide once per 8 hours was injected subcutaneously to inhibit pancreatin secretion until hemodiastase level dropped to normal (medication should be extended in pancreas leakage patients).
Complications criteria
PF criteria: Bassi (19) grade B and C was defined as PF, and grade A was excluded due to its light manifestation and non-special treatment. Biliary fistula criteria: bile-like liquid was observed in abdominal cavity drainage tube among the first 3 days after operation, and flow discharge was above 50 mL/d in 3 continuous days. Postoperative abdominal cavity or alimentary tract hemorrhage criteria: we referred to International Study Group of Pancreatic Surgery (ISGPS) definition (24). Postoperative infection criteria: hemogram was above upper normal level, combining with body temperature higher than 38.5 centigrade, meanwhile, positive outcome in body fluid cultivation, such as blood, abdominal fluid, sputum or bile. Delayed gastric emptying criteria (25): nasogastric tube was detained more than 10 days, and combining with at least one of the following conditions: (I) vomiting after pulling out gullet; (II) using propulsives more than 10 days after operation; (III) inserting nasogastric tube again to decompress; (IV) not able to resume oral intake; patients whose nasogastric tube was detained less than 10 days but suffered at least two of the above conditions, and were confirmed by alimentary tract iodine visualization or upper abdominal CT were also diagnosed gastric emptying disorder.
Statistical analysis
Continuous data were expressed as mean ± SD. Comparison of categorical and continuous variables were performed using χ2 test (or Fisher exact test where appropriate) and Student’s t-test, respectively. Univariate and multivariate sequential analysis of risk factors for GDAS hemorrhage were performed using the binary logistic regression analysis. A P value<0.05 was considered statistically significant. Statistical analysis was performed using SPSS version 18.0 (SPSS inc., Chicago, IL., USA).
Results
Perioperative comparison of two groups
Before operations, there were 26 (9.3%) patients without overt symptom, 220 (78.6%) with obstructive jaundice, 147 (52.5%) with abdominal pain, 113 (40.4%) jaundice combining with abdominal pain, and 34 (12.1%) with obvious weight loss (≥5 kg). Before operations, there were 67 (23.9%) hepatitis B infectors, 27 (9.6%) patients with type 2 diabetes, 22 (7.9%) patients with hypertensions, 5 (1.8%) patients with hepatic cyst, 3 (1.1%) patients with hepatic haemangioma, 3 (1.1%) patients with chronic superficial gastritis and ulcers, two (0.7%) patients with gallbladder stones, 2 (0.7%) patient with renal cyst, 1 (0.4%) patient with intrahepatic bile duct stone, 1 (0.4%) patient with schistosomiasis hepatic cirrhosis, 1 (0.4%) patient with chronic pancreatitis, 1 (0.4%) patient with bronchopneumonia, 1 (0.4%) patient with asthma, 1 (0.4%) patient with gout, 1 (0.4%) patient with depression, 1 (0.4%) patient with neuroma and 1 (0.4%) patient with left eye blindness. A total of 25 (8.9%) patients had upper abdominal surgery history. There were no significant differences between wrapping group and non-wrapping group on age, sex, preoperative manifestations and examination results, preoperative jaundice treatment, size of tumor, pathological diagnosis, main concomitant diseases, upper abdominal operation history, intra-operative hemorrhage volume and hospitalization time (Detailed in Table 1).
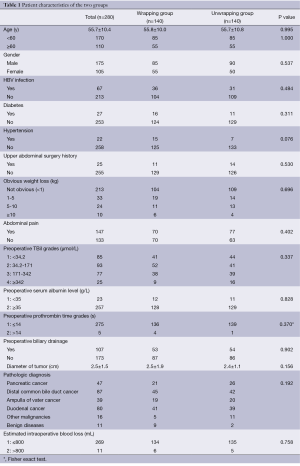
Full table
Complications of two groups
A total of 133 (47.5%) patients suffered from postoperative complications: 47 (16.8%) patients got two or more kinds of complications. There was no significant difference between wrapping group and unwrapping group in the incidence of total complications (69/140 vs. 64/140, P=0.550). The gastroduodenal stump massive hemorrhage rate was significantly lower in wrapping group than that in non-wrapping group (1/140 vs. 9/140, P=0.010); Meanwhile, no significant difference was observed between two groups on other complications, for example, other reasons intra-abdominal massive hemorrhage (except GDAS bleeding) (4/140 vs. 1/140, P=0.370), gastrointestinal massive hemorrhage (14/140 vs. 10/140, P=0.393), intra-abdominal infection (37/140 vs. 26/140, P=0.115), PF (7/140 vs. 14/140, P=0.112), billiary fistula (2/140 vs. 3/140, P=0.652) and delayed gastric emptying (7/140 vs. 13/140, P=0.164). Complications relating with teres hepatis ligamentum wrapping GDAS, such as hepatic arteriostenosis, hepatophyma etc. didn’t occur to all patients in wrapping group. Seven patients (2.5%) accepted reoperation: one for GDAS hemorrhage, one for left gastric artery hemorrhage, one for retroperitoneum hemorrhage, two for gastrointestinal hemorrhage and two for delayed wound healing. Six patients (6/7) recovered after reoperation, and one patient (1/7) who accepted reoperation for gastrointestinal bleeding died. There was no significant difference between wrapping group and unwrapping group on the reoperation rate (5/140 vs. 2/140, P=0.447). Five (3.11%) patients died within 60 days after operations. Two patients died of postoperative GDAS hemorrhage, two patients died of postoperative gastrointestinal bleeding, and one patient died of postoperative heart failure. The rate of postoperative mortality (2/140 vs. 3/140, P=1.000) was unanimous statistically (Detailed in Table 2).
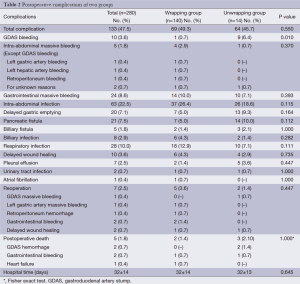
Full table
Treatment and prognosis of GDAS hemorrhage
The GDAS hemorrhage occurrence time of ten patients were at least one week (range from 8 to 43 days) after operations. Digital selective angiography (DSA) and transcatheter arterial embolization (TAE) were performed to stanch bleeding for all of ten GDAS hemorrhage patients, and seven (7/10) of them got successful hemostasis. One patient got hemorrhage volume decreased, and underwent emergent surgical hemostasis successfully after his shock was eased, but the other two kept bleeding after DSA + TAE, and shock were even aggravated, thus emergent surgical hemostasis could not be performed and these two patients died as a result (Detailed in Table 3).
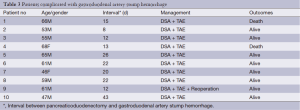
Full table
Discussion
“Wrapping” reduced the GDAS hemorrhage incidence after PD
Traced back to the 20th century, with the invention and widely utilization of somatostatin and octreotide, the incidence of PF and postoperative intra-abdominal hemorrhage relating to PF have been decreased obviously (26,27). In recent years, retrospective or RCT researches on improving pancreatic juice drainage (28-32) and pancreatic-enteric anastomosis (33-42) have been done all over the world, in order to further reduce the PF incidence. However, none of these methods have been demonstrated significant superiority (43), and Peng et al. (20) considered that due to the injuries on pancreatic parenchyma and minor ductus pancreaticus caused by needle and thread during pancreatic-enteric anastomosis, PF is inevitable, and slight PF evokes severe PF. Now the overall incidence of PF is still about 2-22% (1-19), and the incidence of PF who need clinical treatment according to Bassi grading criteria in this study is 7.5%, which is correspondent with former reports. After hepatoduodenal ligament lymph node dissection during PD, GDAS is exposed nearby pancreas-intestinal stomas, and is easily got corroded by pancreatic juice, thus causing a high risk of hemorrhage. Although PF is not the direct death cause, GDAS hemorrhage is possibly fatal. The intra-abdominal hemorrhage rate after PD is approximately 5-16% according to report (44), among which GDAS is a frequent bleeding locus (3-6,8-13,22), and PF and intra-abdominal infection are key risk factors of intra-abdominal hemorrhage (2-4,7-13). In our study, the total postoperative intra-abdominal hemorrhage rate was 5.4% (15/280), but GDAS hemorrhage took up 66% (10/15) of them, which is similar to former results by other scholars.
PF is difficult to avoid completely (20), thus the risk of GDAS corroded by pancreatic juice can not be avoided completely. Some scholars (21,22,45) began to use the omentum or falciform ligament to cover/wrap the exposed major blood vessels, in order to protect the vessels from pancreatic juice and reduce the incidence of postoperative intra-abdominal bleeding. For example, Maeda et al. indicated only one patient (1/100) occurred postoperative intra-abdominal bleeding after covering the vessels using omental flap during PD (21); Abe et al. reported none patient (0/36) developed late post-pancreatectomy hemorrhage after the pedicled falciform ligament was used to cover the major exposed vessels, and was fixed to the surrounding retroperitoneal connective tissue (45); Sakamoto et al. reported just one patient (1/136) developed GDAS hemorrhage after wrapping the GDAS using the falciform ligament (22). In our study, only one patient (1/140) developed GDAS hemorrhage after GDAS wrapping by teres hepatis ligamentum, which was similar with the outcome in single arm, non-control group clinical research by Maeda et al., Sakamoto et al. and Abe et al. (21,22,45). We adopted the non-wrapping patients as control, and found that GDAS hemorrhage incidence was significantly lower in wrapping group than in non-wrapping group (1/140 vs. 9/140, P=0.010), which indicated that wrapping GDAS is beneficial to reduce GDAS bleeding incidence. On the contrary, a recent retrospective study of the Japanese Society of Pancreatic Surgery indicated that using omentum or falciform ligament did not decrease the incidence of intra-abdominal hemorrhage after PD (23). On one hand, the study adopted polycentric retrospective data, while statistical bias may occur for the fact that the operation standard differed from each center and researchers cumulated the data from different centers directly; on the other hand, whether wrapping GDAS can intrinsically reduce GDAS hemorrhage incidence is still under debate, and RCT study is an urgent need in order to find out the value of wrapping GDAS.
“Wrapping” had no obvious influence on other complications
For instance, no significant difference was observed on the postoperative PF incidence in two groups (7/140 vs. 14/140, P=0.112), which is similar to former results (21-23,45). Maeda et al. advocated that wrapping porta hepatic blood vessel by omentum majus could reduce the incidence of postoperative intra-abdominal infection after PD (21), while our data did not show significant difference on intra-abdominal infection between two groups (37/140 vs. 26/140, P=0.115). There were no such complications as hepatic artery stenosis, hepatic function recovery disorder, and hepatophyma that relating to the wrapping procedure in the wrapping group, which was similar to the results obtained by Maeda et al. (21) and Abe et al. (45).
GDAS should be entirely “wrapped” with gentle approach
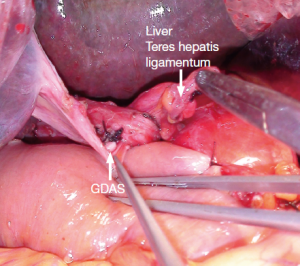
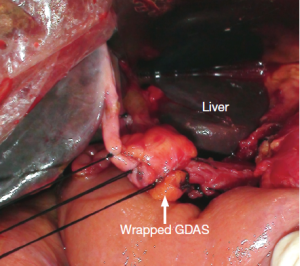
The approach we adopted to wrap GDAS is similar to that reported by Sakamoto et al. (22) (Figure 1). However, they wrapped GDAS by the pedicled falciform ligament, and we chose the pedicled teres hepatis ligamentum. Although the procedure of wrapping GDAS is simple and low time-consuming, there are two key points that should be noticed: (I) GDAS should be wrapped entirely to separate from the site of pancreatojejunostomy; (II) wrapping should not be too tight to affect the hepatic artery blood supply (Figures 1,2). Our data showed: there were no significant differences between wrapping group and non-wrapping group on the other postoperative complications (except GDAS hemorrhage incidence).
DSA + TAE was useful for early stage of GDAS hemorrhage, but once hemodynamic instability occurred emergency surgical hemostasis might be more profitable
Due to the fact that gastroduodenal artery (GDA) has a crude caliber, and once hemorrhea happens, it arouses shock and life-threatening result if bleeding cannot be controlled promptly and effectively. Now that medical treatment cannot stop bleeding effectively, DSA + TAE or emergent surgical hemostasis are the possible approaches to rescue lives. Sato et al. considered it is vital to perform early angiography in patients with intra-abdominal hemorrhage (13). Choi et al. indicated TAE provides not only a basic treatment, but also a temporary hemostatic effect that makes it easy to reoperate if necessary (4). But, Tien et al. considered that TAE could not be safely performed after hemodynamic instability occurred, so they performed surgical hemostasis on 70% GDAS hemorrhage patients (46). According to this study we performed DSA + TAE treatment in all of ten GDAS bleeding patients. Seventy percent (7/10) of them got successful hemostasis. One patient (10%) got hemorrhage volume decreased, after his shock was eased, emergent surgical hemostasis performed successfully. But 20% (2/10) of them failed, both of them accepted DSA + TAE treatment with unstable hemodynamics and died (shock was even aggravated after DSA + TAE treatment, and the chances of the emergent surgical hemostasis were lost. Thus, we considered that DSA + TAE treatment might be extremely useful for early stage of GDAS hemorrhage patients, but once hemodynamic instability occurred DSA + TAE treatment might not be profitable and emergency surgical hemostasis should be taken as soon as quickly.
Acknowledgements
This study was funded by A new round of the Shanghai Health System outstanding young talent training plan (XYQ2011030).
Disclosure: The authors declare no conflict of interest.
References
- Cullen JJ, Sarr MG, Ilstrup DM. Pancreatic anastomotic leak after pancreaticoduodenectomy: incidence, significance, and management. Am J Surg 1994;168:295-8. [PubMed]
- Rumstadt B, Schwab M, Korth P, et al. Hemorrhage after pancreatoduodenectomy. Ann Surg 1998;227:236-41. [PubMed]
- Brodsky JT, Turnbull AD. Arterial hemorrhage after pancreatoduodenectomy. The ‘sentinel bleed’. Arch Surg 1991;126:1037-40. [PubMed]
- Choi SH, Moon HJ, Heo JS, et al. Delayed hemorrhage after pancreaticoduodenectomy. J Am Coll Surg 2004;199:186-91. [PubMed]
- Camerlo A, Turrini O, Marciano S, et al. Delayed arterial hemorrhage after pancreaticoduodenectomy: is conservation of hepatic arterial flow vital? Pancreas 2010;39:260-2. [PubMed]
- Blanc T, Cortes A, Goere D, et al. Hemorrhage after pancreaticoduodenectomy: when is surgery still indicated? Am J Surg 2007;194:3-9. [PubMed]
- Koukoutsis I, Bellagamba R, Morris-Stiff G, et al. Haemorrhage following pancreaticoduodenectomy: risk factors and the importance of sentinel bleed. Dig Surg 2006;23:224-8. [PubMed]
- Shankar S, Russell RC. Haemorrhage in pancreatic disease. Br J Surg 1989;76:863-6. [PubMed]
- van Berge Henegouwen MI, Allema JH, van Gulik TM, et al. Delayed massive haemorrhage after pancreatic and biliary surgery. Br J Surg 1995;82:1527-31. [PubMed]
- Makowiec F, Riediger H, Euringer W, et al. Management of delayed visceral arterial bleeding after pancreatic head resection. J Gastrointest Surg 2005;9:1293-9. [PubMed]
- Yamashita Y, Taketomi A, Fukuzawa K, et al. Risk factors for and management of delayed intraperitoneal hemorrhage after pancreatic and biliary surgery. Am J Surg 2007;193:454-9. [PubMed]
- de Castro SM, Kuhlmann KF, Busch OR, et al. Delayed massive hemorrhage after pancreatic and biliary surgery: embolization or surgery? Ann Surg 2005;241:85-91. [PubMed]
- Sato N, Yamaguchi K, Shimizu S, et al. Coil embolization of bleeding visceral pseudoaneurysms following pancreatectomy: the importance of early angiography. Arch Surg 1998;133:1099-102. [PubMed]
- Matsumoto Y, Fujii H, Miura K, et al. Successful pancreatojejunal anastomosis for pancreatoduodenectomy. Surg Gynecol Obstet 1992;175:555-62. [PubMed]
- Yeo CJ, Cameron JL, Sohn TA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg 1997;226:248-57; discussion 257-60. [PubMed]
- Büchler MW, Friess H, Wagner M, et al. Pancreatic fistula after pancreatic head resection. Br J Surg 2000;87:883-9. [PubMed]
- Schmidt CM, Powell ES, Yiannoutsos CT, et al. Pancreaticoduodenectomy: a 20-year experience in 516 patients. Arch Surg 2004;139:718-25; discussion 725-7. [PubMed]
- Cameron JL, Riall TS, Coleman J, et al. One thousand consecutive pancreaticoduodenectomies. Ann Surg 2006;244:10-5. [PubMed]
- Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 2005;138:8-13. [PubMed]
- Peng SY, Wang JW, Lau WY, et al. Conventional versus binding pancreaticojejunostomy after pancreaticoduodenectomy: a prospective randomized trial. Ann Surg 2007;245:692-8. [PubMed]
- Maeda A, Ebata T, Kanemoto H, et al. Omental flap in pancreaticoduodenectomy for protection of splanchnic vessels. World J Surg 2005;29:1122-6. [PubMed]
- Sakamoto Y, Shimada K, Esaki M, et al. Wrapping the stump of the gastroduodenal artery using the falciform ligament during pancreaticoduodenectomy. J Am Coll Surg 2007;204:334-6. [PubMed]
- Tani M, Kawai M, Hirono S, et al. Use of omentum or falciform ligament does not decrease complications after pancreaticoduodenectomy: nationwide survey of the Japanese Society of Pancreatic Surgery. Surgery 2012;151:183-91. [PubMed]
- Wente MN, Veit JA, Bassi C, et al. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery 2007;142:20-5. [PubMed]
- Yeo CJ, Barry MK, Sauter PK, et al. Erythromycin accelerates gastric emptying after pancreaticoduodenectomy. A prospective, randomized, placebo-controlled trial. Ann Surg 1993;218:229-37; discussion 237-8. [PubMed]
- Connor S, Alexakis N, Garden OJ, et al. Meta-analysis of the value of somatostatin and its analogues in reducing complications associated with pancreatic surgery. Br J Surg 2005;92:1059-67. [PubMed]
- Alghamdi AA, Jawas AM, Hart RS. Use of octreotide for the prevention of pancreatic fistula after elective pancreatic surgery: a systematic review and meta-analysis. Can J Surg 2007;50:459-66. [PubMed]
- Roder JD, Stein HJ, Böttcher KA, et al. Stented versus nonstented pancreaticojejunostomy after pancreatoduodenectomy: a prospective study. Ann Surg 1999;229:41-8. [PubMed]
- Imaizumi T, Hatori T, Tobita K, et al. Pancreaticojejunostomy using duct-to-mucosa anastomosis without a stenting tube. J Hepatobiliary Pancreat Surg 2006;13:194-201. [PubMed]
- Winter JM, Cameron JL, Campbell KA, et al. Does pancreatic duct stenting decrease the rate of pancreatic fistula following pancreaticoduodenectomy? Results of a prospective randomized trial. J Gastrointest Surg 2006;10:1280-90; discussion 1290. [PubMed]
- Ohwada S, Tanahashi Y, Ogawa T, et al. In situ vs ex situ pancreatic duct stents of duct-to-mucosa pancreaticojejunostomy after pancreaticoduodenectomy with billroth I-type reconstruction. Arch Surg 2002;137:1289-93. [PubMed]
- Poon RT, Fan ST, Lo CM, et al. External drainage of pancreatic duct with a stent to reduce leakage rate of pancreaticojejunostomy after pancreaticoduodenectomy: a prospective randomized trial. Ann Surg 2007;246:425-33; discussion 433-5. [PubMed]
- Hosotani R, Doi R, Imamura M. Duct-to-mucosa pancreaticojejunostomy reduces the risk of pancreatic leakage after pancreatoduodenectomy. World J Surg 2002;26:99-104. [PubMed]
- Langrehr JM, Bahra M, Jacob D, et al. Prospective randomized comparison between a new mattress technique and Cattell (duct-to-mucosa) pancreaticojejunostomy for pancreatic resection. World J Surg 2005;29:1111-9; discussion 1120-1. [PubMed]
- Lee SE, Yang SH, Jang JY, et al. Pancreatic fistula after pancreaticoduodenectomy: a comparison between the two pancreaticojejunostomy methods for approximating the pancreatic parenchyma to the jejunal seromuscular layer: interrupted vs continuous stitches. World J Gastroenterol 2007;13:5351-6. [PubMed]
- Bassi C, Falconi M, Molinari E, et al. Duct-to-mucosa versus end-to-side pancreaticojejunostomy reconstruction after pancreaticoduodenectomy: results of a prospective randomized trial. Surgery 2003;134:766-71. [PubMed]
- Z'graggen K, Uhl W, Friess H, et al. How to do a safe pancreatic anastomosis. J Hepatobiliary Pancreat Surg 2002;9:733-7. [PubMed]
- Ibrahim S, Tay KH, Launois B, et al. Triple-layer duct-to-mucosa pancreaticojejunostomy after pancreaticoduodenectomy. Dig Surg 2006;23:296-302. [PubMed]
- Hayashibe A, Kameyama M. The clinical results of duct-to-mucosa pancreaticojejunostomy after pancreaticoduodenectomy in consecutive 55 cases. Pancreas 2007;35:273-5. [PubMed]
- Murakami Y, Uemura K, Hayashidani Y, et al. No mortality after 150 consecutive pancreatoduodenctomies with duct-to-mucosa pancreaticogastrostomy. J Surg Oncol 2008;97:205-9. [PubMed]
- Peng S, Mou Y, Cai X, et al. Binding pancreaticojejunostomy is a new technique to minimize leakage. Am J Surg 2002;183:283-5. [PubMed]
- Chen HW, Lai EC, Su SY, et al. Modified technique of pancreaticojejunal anastomosis with invagination following pancreaticoduodenectomy: a cohort study. World J Surg 2008;32:2695-700. [PubMed]
- Lai EC, Lau SH, Lau WY. Measures to prevent pancreatic fistula after pancreatoduodenectomy: a comprehensive review. Arch Surg 2009;144:1074-80. [PubMed]
- Balachandran P, Sikora SS, Raghavendra Rao RV, et al. Haemorrhagic complications of pancreaticoduodenectomy. ANZ J Surg 2004;74:945-50. [PubMed]
- Abe N, Sugiyama M, Suzuki Y, et al. Falciform ligament in pancreatoduodenectomy for protection of skeletonized and divided vessels. J Hepatobiliary Pancreat Surg 2009;16:184-8. [PubMed]
- Tien YW, Lee PH, Yang CY, et al. Risk factors of massive bleeding related to pancreatic leak after pancreaticoduodenectomy. J Am Coll Surg 2005;201:554-9. [PubMed]


