Alpha-2-macroglobulin as a radioprotective agent: a review
Introduction
Radiotherapy is the most common modality in cancer treatment. An estimated eighty percent of cancer patients need radiotherapy at some time or other, either for curative or palliative purposes (1). Radiotherapy is frequently used to obtain local or regional control of malignancies either alone or in combination with other modalities such as chemotherapy or surgery (2). However, exposure of normal tissue to radiation may lead to radiation-induced damage that can result in the inability to deliver the intended therapy, a range of symptoms, and a decrease in quality of life. Irradiation of noncancerous “normal” tissues during the course of therapeutic radiation can result in a range of side effects including self-limited acute toxicities, mild chronic symptoms, or severe organ dysfunction. Thus, radiation-induced damage to the normal tissues restricts the therapeutic doses of radiation that can be delivered to tumours and thereby limits the effectiveness of the treatment. To achieve better cancer control and possible cure, a judicious balance between the total dose of radiotherapy delivered and the threshold limit of the surrounding normal critical tissues is required. To obtain optimal results, the normal tissues should be protected against radiation injury. Hence, the role of radioprotective compounds is of great importance in clinical radiotherapy (3).
Radioprotectors are synthetic compounds or natural products that are immediately administered before irradiation to reduce injuries caused by ionising radiation. Over the past 60 years, as a result of the great clinical need for effective radioprotectant agents, many have set out to find more effective, less toxic drugs. Initial attempts focused on synthetic thiol compounds. These agents are highly effective at reducing lethality induced by irradiation. Of this class, amifostine is the only radioprotector that has been clinically approved by the Food and Drug Administration (FDA) for mitigating side effects (xerostomia) in patients undergoing radiotherapy (4,5). This drug offers good protection, but is relatively toxic (nausea, vomiting and hypotension being some of the most common adverse effects) (6,7). In view of this, there is still an urgent need to identify novel, nontoxic, effective, and convenient compounds to protect humans from damaging effects of ionising radiation. Aside from synthetic compounds, some safe and effective naturally occurring products that produce a nonspecific response in the body that increases the power of resistance, function as antioxidants and immunostimulants and restore homeostasis-stimulated radioresistance have also been included in the category of radioprotectors (1). Studies in the recent past have shown that alpha-2-macroglobulin (α2M) possesses radioprotective effects. This review focuses on the observations and elucidates the possible mechanisms responsible for the radioprotective effects of α2M.
Radiation injury and radioprotection
The deleterious effects of ionising radiation are mediated through direct deposition of energy to biological molecules and indirectly through generation of highly reactive free radicals (1). Exposure to ionising radiation induces the production of reactive oxygen species (ROS), which include superoxide, hydroxyl radicals, singlet oxygen, and hydrogen peroxide. Free radicals react with DNA, RNA, proteins, and membranes, resulting in cell dysfunction and death. Radiation sickness, also referred to as the acute radiation syndrome (ARS), is a serious illness that occurs when the entire body (or most of it) receives a high dose of radiation over a short period of time (8). Because ionising radiation causes cell dysfunction and mortality, extensive research is devoted to the development of effective radioprotective compounds (9) that would diminish radiation injury in living organisms (1).
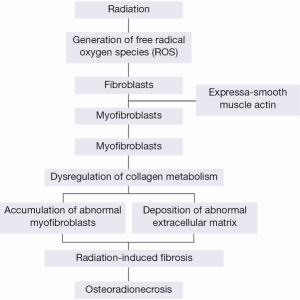
Radiation-induced fibrosis is a new theory that accounts for the damage to normal tissues, including bone, after radiotherapy (10). The establishment of radiation-induced fibrosis involves free radical production immediately after the irradiation, resulting in complex multistep activation processes that persist for months and years. This pathology is characterised by the deposition of newly formed extracellular matrix components resulting from an altered balance between connective tissue production and degradation, and by the accumulation of specific fibroblastic cells called myofibroblasts that exhibit contractile properties (11,12). Myofibroblasts are defined as transiently activated fibroblasts exhibiting features intermediate between those of smooth muscle cells and fibroblasts, including the expression of α-smooth muscle actin (α-SM actin) (13,14) and a depleted antioxidant system (15). These cells were introduced in 2004 when recent advances in cellular and molecular biology explained the progression of microscopically observed ORN (Figure 1) (16).
The theory of radiation-induced fibrosis suggests that the key event in the progression of ORN is the activation and dysregulation of fibroblastic activity that leads to atrophic tissue within a previously irradiated area. After radiotherapy the endothelial cells are injured, both from direct damage by radiation and from indirect damage by radiation-generated ROS or free radicals. Injured endothelial cells produce chemotactic cytokines that trigger an acute inflammatory response and then generate a further release of ROS from polymorphs and other phagocytes (17). The destruction of endothelial cells, coupled with vascular thrombosis, lead to necrosis of micro-vessels, local ischaemia, and tissue loss. Loss of the natural endothelial cell barrier allows seepage of various cytokines that cause fibroblasts to become myofibroblasts. The ROS-mediated release of cytokines such as tumour necrosis factor β (TNF-β), platelet-derived growth factor, fibroblast growth factor α, interleukins 1, 4, and 6, transforming growth factor α1 (TGF-α1), and connective tissue growth factor, result in unregulated fibroblastic activation and the myofibroblast phenotype persists (17).
Because exposure to irradiation in radiotherapy or accidental exposure to radiation can produce significant unwanted side effects, it is important to ameliorate such effects by the use of radioprotective drugs (18). The radioprotectors can elicit their action by various mechanisms, such as: (I) suppressing the formation of reactive species; (II) detoxification of radiation induced species; (III) target stabilisation; and (IV) enhancing the repair and recovery processes (19). The ideal radioprotective agent should fulfil several criteria including significant protection against the effects of radiation, a general protective effect on the majority of organs, an acceptable route of administration, an acceptable toxicity profile and protective time-window effect, an acceptable stability profile and compatibility with the wide range of other drugs (20). Unfortunately, to date, there is no radioprotector that fulfils all of these criteria. A search for alternative agents that are less toxic and highly effective is of great importance.
Alpha-2-macroglobulin (α2M) as a radioprotective agent
Human α2M is a 720-kDa glycoprotein in which five reactive sites have been characterised in each of its four identical subunits (180 kDa) (21). α2M is the largest major non-immunoglobulin protein in plasma. The α2M molecule is synthesised by numerous cell lineages, including lung fibroblasts, monocytes-macrophages, hepatocytes, astrocytes and adrenocortical cells (22-24).
In cultured bovine adrenocortical cells, synthesis of α2M may be selectively and significantly increased by TGF-β (25). Interleukin-6 (IL-6) induces α2M synthesis by human neuronal cells, a mechanism of possible significance in some diseases of the central nervous system (26). A significant increase in α2M plasma levels is consistently observed during embryogenesis, pregnancy, and childhood, all representing periods of life characterised by growth, development, and differentiation (23,27-29). α2M is a tetrameric, disulfide-rich plasma glycoprotein with several functions (22). Some of its functions, such as inhibition of different types of nonspecific proteases and transport of cytokines, growth factors, and hormones and a pronounced immune-suppressive activity (30,31), are well established.
α2M is a pan-proteinase inhibitor capable of inhibiting a large variety of proteinases. After proteolytic cleavage of the “bait” region, the proteinase is entrapped and loses most of its activity, at least toward high molecular weight substrates (22). The concomitant interruption of the thiolester triggers further biological functions of the inhibitor such as binding cytokines, growth factors and hormones (32,33), as well as clearance by the α2M-R/LRP present on the surface of different cells (34-36). The α2MR/LRP is a 600-kDa glycoprotein that undergoes proteolysis in the trans-Golgi and is expressed as a non-covalently associated heterodimer of 515 and 85 kDa, respectively (30). The α2M-R/LRP is expressed in hepatocytes where it plays an important role in the regulation of proteolytic activity in tissue and peri-cellular space and contributes mainly to the clearance of α2M-proteinase complexes from circulation (37).
Recent studies have shown that α2M possesses radioprotective effects. Evidence implicates the α2-macroglobulin fraction (19S) (containing α2M) in the recovery of mice from radiation damage. The α2- macroglobulin fraction has been shown to enhance the regeneration of haematopoietic cells (38) and lymphopoietic cells (39) in X-irradiated mice. The haematopoietic system is among the most radiosensitive tissues in the body.
α2M most likely plays a role in maintaining hemodynamic equilibrium because the primary cause of death after repeated scalding is circulatory shock provoked by a decrease in plasma volume of more than 50% (40). This is supported by the finding that α2M inhibits the activity of prostaglandin E2, which increases the permeability of blood vessels (41) and acts as a vasodilator (42). Sevaljević et al. reported that the administration of the rat acute-phase (AP) protein a2-macroglobulin (α2M) that results in a 15-fold increase in its concentration from the normal basal level, 30 min either before the infliction of a lethal scalding (43) or exposure to total body irradiation with 6.7 Gy (LD50/30) X-rays (44,45) enabled 100% survival in experimental animals. Indeed, pretreatment with α2M before whole-body irradiation allowed for the restoration of body weight, leukocyte count, the complete recovery of liver mass and fully protected liver morphology during a four week follow-up period covering the duration of the ARS. Otherwise, pretreatment with α2M also induced a significant reduction of irradiation-induced DNA damage and the complete restoration of liver and body weight. Mihailović et al. conclude that the radioprotection provided by α2M was in part mediated through cytoprotection of new blood cells produced in the bone marrow and they also indicate that an important aspect of the radioprotective effect of amifostine is the result of induction of the endogenous cytoprotective capability of α2M (45,46). Mihailović et al. [2009] compare the cytoprotective effects of α2M and amifostine on rat liver. At 2 weeks after irradiation, Comet assays revealed a 15-fold increase in DNA damage in unprotected rats, while in α2M- and amifostine-treated rats we observed 3- and 4-fold rise in damage, respectively (45).
Bogojević et al. [2011] reported that compared to untreated rats, pretreatment with α2M and amifostine led to similar increases in levels of the inflammatory cytokine IL-6 and the redox-sensitive transcription factor NFκB, promoting upregulation of MnSOD, the major component of the cell’s antioxidant axis, and subsequent increases in Mn/CuZnSOD and catalase enzymatic activities. The results show that α2M induces protein factors whose interplay underlies radioprotection and support the idea that α2M is the central effector of natural radioprotection in the rat (47).
Mechanisms responsible for the radioprotection of alpha-2-macroglobulin (α2M)
The mechanisms responsible for the radioprotective effects of α2M can be summarised in five aspects as follows: antioxidant, anti-fibrosis, anti-inflammatory, maintaining homeostasis and enhancement of the DNA repair and cell recovery processes. We will elucidate the mechanisms responsible for the radioprotective effects of α2M in detail point by point below.
Alpha-2-macroglobulin (α2M) functions as an antioxidant
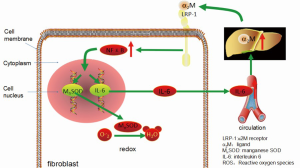
Ionising radiation causes immediate direct damage to macromolecules and also generates ROS in irradiated tissues and cells (48). The antioxidant system in the liver includes enzymatic and non-enzymatic components that control the flux of ROS (48). The first line of defence against increased levels of ROS is provided by the activities of the major antioxidant enzymes such as superoxide dismutase (SODs) that inactivates the superoxide anion by catalysing its dismutation into oxygen and hydrogen peroxide, and catalase that inactivates the peroxide (49). The enhancement of endogenous intracellular antioxidant enzyme levels can be very important in the adaptive, inherent radiation resistance of cells. An example is manganese superoxide dismutase (MnSOD), which was identified as the most effective endogenous antioxidant enzyme in protecting against radiation-induced cell toxicity (50). Bogojević et al. [2011] reported that pretreatment with α2M led to increased levels of the inflammatory cytokine IL-6 and the redox-sensitive transcription factor NFκB (p65), promoting upregulation of MnSOD, the major component of the cell’s antioxidant axis, and subsequent increases in Mn/CuZnSOD and catalase enzymatic activities (47). As α2-macroglobulin is a carrier protein for IL-6, the powerful inducer of its synthesis, these two proteins can modulate each other’s activity. It does not inhibit IL-6 activity or its binding to homologous receptor. In addition, IL-6 bound to α2M is resistant to proteinases, whereas free IL-6 is easily degraded (51). NFκB is predominantly comprised of p65/p50 heterodimers that are sequestered in the cytoplasm by association with members of the IκB protein family, which bind NFκB and thereby mask its nuclear localisation signal. Upon stimulation by inflammatory cytokines or DNA damage, IκB molecules are degraded, promoting the nuclear translocation of NFκB and binding to target genes. Only p65 and c-Rel activate transcription (52). Pretreatment with α2M led to increased levels of NFκB (p65), increased nuclear transcription signals, and activation target genes (e.g., MnSOD gene) resulting in increased protein expression. SOD enzymes are naturally occurring intracellular enzymes, which scavenge O2– by catalysing its conversion to hydrogen peroxide and oxygen. It has become clear that these enzymes provide an essential defence against the superoxide radical. The copper-, zinc- and manganese-containing SODs (Cu, Zn, Mn and SOD) are the most common type of SOD (53,54). Mn/CuZnSOD and catalase are naturally occurring antioxidant enzymes and α2M lead to subsequent increases in Mn/CuZnSOD and catalase enzymatic activities and then the ability of free radical scavenging increases.
Combined with radiation-induced fibrosis theory, the antioxidant effect of α2M on radiation injury may be as follows (Figure 2). α2M binds LRP-1 on the fibroblast membrane and sends the signal to NFκB and promotes the nuclear translocation of NFκB and binds to target genes (MnSOD and IL-6 genes), and then the expression of MnSOD and IL-6 increases. MnSOD scavenges O2– by catalysing its conversion to hydrogen peroxide and oxygen. IL-6 enters the blood circulation and reaches the liver to induce the synthesis of α2M. Then, α2M binds LRP-1 on the fibroblast membrane again.
Anti-fibrosis
The theory of radiation-induced fibrosis suggests that the key event in the progression of its pathophysiology is the activation and dysregulation of fibroblastic activity that leads to atrophic tissue within a previously irradiated area. Activated fibroblasts, namely myofibroblasts, exhibit features intermediate between those of smooth muscle cells and fibroblasts, including the expression of a-smooth muscle actin (a-sm actin) (13,14), and a depleted antioxidant system (15).
To impede the pathological process of radiation-induced fibrosis, we can block the activation of fibroblasts to myofibroblasts to prevent fibrosis. The ROS-mediated release of cytokines such as TNF-β, platelet-derived growth factor (PDGF), fibroblast growth factor α (FGF-α), interleukins 1, 4, and 6 (IL-1, 4, 6), TGF-α1, and connective tissue growth factor result in unregulated fibroblastic activation and the myofibroblast phenotype persists (17). α2M binds different acute inflammatory mediators (TNF-α, IL-1β, IL-2, IL-6) and growth factors (β-nerve growth factor, platelet-derived growth factor BB, transforming growth factor β1 (TGF-β1), transforming growth factor β2 (TGF-β2)), extremely potent hormone-like polypeptides that play essential roles in the regulation of cellular functions (22,30,55). One aspect of the physiological role of α2M-cytokine/α2M-growth factor binding is the downregulation of their activities because bound polypeptides lose their ability to regulate cell functions, whereas the abilities of others become enhanced (56). Thus, α2M binds cytokines such as TGF-β, PDGF, FGF-α, IL-1, -4, -6, downregulates their activities, and then block the process of activating fibroblasts to become myofibroblasts.
Anti-inflammatory ability
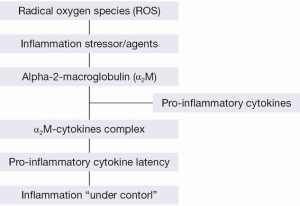
Under conditions of chronic oxidative stress, as would be encountered in irradiated tissue, the generation of ROS triggers an inflammatory response through the activation of cytokines and other inflammatory mediators (57). Indeed, it has been shown that the decreased T-cell response is the consequence of IL-2 degradation and inactivation by α2M-bound proteinases (58). Other cytokines, such as pro-inflammatory cytokines (IL-1, IL-6 and TNF-α), bind α2M without being degraded (58). The formation of the α2M–pro-inflammatory cytokine complex has been suggested as protection from the immediate toxic effect of cytokines, protection from extracellular proteolysis and the loss of cytokines through the kidney and, finally, as a mechanism to target cytokines to α2M-receptor-bearing cells (58). These phenomena occur as indirect or alternative inhibitory roles of α2M against increased MMPs, which are in turn temporarily produced in response to exogenous signals by pro-inflammatory cytokines (59).
The binding of α2M with pro-inflammatory cytokines occurs to induce latency of cytokines themselves (58). As a consequence, inflammation is ‘‘under control’’ (Figure 3) (55). In addition, the possible overexpression of these cytokines may also be avoided by the α2M-cytokine complex through α2MR/LRP (60). Proinflammatory cytokines (IL-1, IL-6 and TNF-α) increase during inflammation (61), and IL-6 regulates the α2M gene expression (62). Thus, the interrelationship between α2M and the immune system is evident with a role of protection against toxic effects by an over production of pro-inflammatory cytokines during inflammation.
Maintaining homeostasis
The pathology of radiation-induced fibrosis is characterised by the deposition of newly formed extracellular matrix components resulting from an altered balance between connective tissue production and degradation and by the accumulation of specific fibroblastic cells called myofibroblasts exhibiting contractile properties (11,12).
In addition to anti-fibrosis, maintaining homeostasis is also of great importance.
The significant release of proteinases by activated neutrophils is one important aspect of the potentially negative side effects of inflammatory processes. Thus, as a result of direct damage by radiation and activated defensive mechanisms, the ensuing large-scale cell destruction that occurs is connected to a burst of proteinase activity. Ionising radiation also stimulates proteolysis of proteins by proteases (63). One aspect of the radioprotective efficacy of α2M could be attributed to its unique ability to inhibit all classes of proteinases (22,30,64).
Indeed, several studies have demonstrated the importance of α2M in maintaining the proteinase-proteinase inhibitor equilibrium during inflammation (30). α2-macroglobulin has been generally viewed as an inflammatory fluid proteinase scavenger. α2M is able to inactivate an enormous variety of proteinases (including serine-, cysteine-, aspartic-, and metalloproteinases). α2M has a 35 amino acid “bait” region in its structure. Proteinases binding and cleaving the bait region become bound to α2M. The proteinase-α2M complex is recognised by macrophage receptors and cleared from the system (30).
Irradiation induces primary oxidative damage of biomolecules, including lipids, proteins, and DNA (1). Damage to cellular proteins by oxygen free-radicals formed as a result of action of exogenous factors (radiation, oxidants etc.) underlies the pathogenesis of many diseases (65). Thus, oxidation of amino acid residues in the active centre of enzymes could induce considerable alterations of their catalytic properties and, as a consequence, impair cellular regulatory processes. Ionising radiation also stimulates proteolysis by proteases (63). Interaction between α2M and proteases in the plasma and extracellular fluids involve a unique trapping mechanism by which proteases are incorporated covalently into the α2M molecule, diminishing their proteolytic effect during irradiation.
The application of α2M before whole-body irradiation enables 100% survival in experimental rats and allows for the complete restoration of body weight and leukocyte count by the end of a four-week follow-up period covering the duration of acute radiation injury (46). These results and the findings presented here suggest that α2M, a typical rat AP plasma protein, and functions as a natural radioprotector by stimulating processes that restore homeostasis (44). In view of the important role of α2M in the restoration of homeostasis during the AP response (66,67), we concluded that in the rat α2M behaved as an adaptogen with a radioprotective function.
We also reported that pretreatment with α2M prevented the usual decrease in plasma volume observed after scalding (43). α2M most likely plays a role in maintaining hemodynamic equilibrium because the primary cause of death after repeated scalding is circulatory shock provoked by a decrease in plasma volume of more than 50% (40). This is supported by the finding that α2M inhibits the activity of prostaglandin E2, which increases the permeability of blood vessels (41) and acts as a vasodilator (42). Trocha and Catravas showed that on the third day after irradiation of amifostine-treated rats, prostaglandin concentrations reached a near physiological level (68). This finding, together with the similar degree of radioprotection observed after either the α2M or amifostine pretreatments described here, indicates that α2M affects vascular permeability and promotes normovolemia that in turn protects the organism from the harmful effects of radiation exposure (46).
Enhancement of the DNA repair and cell recovery processes
Evidence has been provided which implicates the α2-macroglobulin fraction (19S) (containing α2M) in the recovery of mice from radiation damage. The α2-macroglobulin fraction has been shown to enhance the regeneration of haematopoietic cells (38), and lymphopoietic cells (39) in X-irradiated mice. Cytological studies of the haematopoietic recovery indicated that the injection of α-globulin fraction into irradiated mice enhanced the recovery, both in time and magnitude, of the granuloid, lymphocyte and lymphocyte-like elements of the bone marrow, as well as of leucocytes in the peripheral blood. The bone marrow, together with lymphoid tissue, the gastrointestinal epithelium, gonads and embryonic tissues, is highly radiosensitive. In the present study, rats from the unprotected irradiated group displayed the highest mortality rate within two weeks of X-irradiation. Up to the end of the 2nd week after irradiation, animals from this group exhibited decreased leukocyte counts, whereas pre-treatment with α2M-pretreatment provided full survival and restoration of leukocyte numbers by the end of the follow-up period (69). In untreated irradiated animals, the number of leukocytes decreased rapidly after irradiation exposure whereas in the α2M-pretreated animals during the followed up period it increased.
Cytokines induce the growth and differentiation of many cells of connective tissue and immune, central nervous, vascular and endocrine systems (70). The multiplicity of important functions that are induced by these cytokines indicates that these agents play essential roles in defensive reactions against infections, in the recovery from injury, and in mediating the radioprotective effect of immunomodulators.
α2M binds different acute inflammatory mediators of which the most important are the cytokines (TNF-α, IL-1β, IL-2, IL-6) and growth factors (β-NGF, PDGF-BB, TGF-β1, TGF-β2) that play essential roles in the regulation of cellular functions that bring about tissue repair and remodelling (55) and participate in mechanism responsible for radioprotection (71,72). Accordingly, α2M has emerged as a dynamic control point for cytokine/growth factor release and their subsequent functioning. It is plausible that the pre-emptive establishment of very high concentrations of α2M in the circulation in α2M-pretreated and pregnant rats provided optimal conditions for highly selective cytokine delivery to target sites of action where repair of irradiation-induced damage was executed (56). Moreover, α2M exerts an antiapoptotic effect (73).
Conclusions
This article reviews different features that make α2M a potentially useful radioprotector such as: the ability to stimulate the activity of antioxidant enzymes; anti-fibrosis effects, namely preventing fibroblasts from becoming myofibroblasts; anti-inflammatory effects, reduction of inflammatory response; maintaining homeostasis and hemodynamic equilibrium; and enhancement of the DNA repair and cell recovery processes.
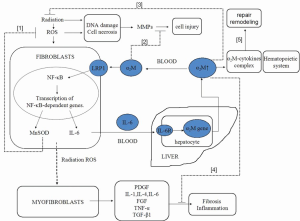
According to the theory of radiation-induced fibrosis (10) and the possible mechanism of the radioprotective effects induced by α2M, we hypothesised that α2M may interact with cytokines and antioxidant enzymes to play a role in radioprotection. The mechanisms are summarised as follows (Figure 4). α2M binds LRP-1 on the fibroblast membrane and activates to NFκB to promote the nuclear translocation of NFκB and transcription of target genes (MnSOD and IL-6 genes), and subsequent increased expression of MnSOD and IL-6 increases. MnSOD scavenges O2– by catalysing its conversion to hydrogen peroxide and oxygen. IL-6 enters the blood circulation and reaches the liver to induce the synthesis of α2M. Then, α2M again binds LRP-1 on the fibroblast membrane. The higher level of α2M plays an important role in radioprotective effects through the mechanisms of anti-fibrosis, anti-inflammatory, antioxidant, homeostasis, and repair and remodelling.
As α2M exists naturally in all mammals, it has a lower toxicity and provides an extended window of protection. Therefore, we propose that in the future, α2M improve the therapeutic ratio in radiation oncology. There is continued interest and need for the identification and development of non-toxic and effective radioprotective compounds. Such compounds could potentially protect humans from the genetic damage, mutagenicity, immune system alterations, and teratogenic effects of toxic agents that act through the generation of free radicals. In hundreds of investigations, α2M appears to provide the desired effect. The optimum dose of α2M for human radioprotection, however, is yet to be determined.
Acknowledgements
I thank Dr. Fang for guidance in related fields and assistance with the English language revision.
Funding: This publication was supported by grant of the Science and Technology Planning Project of Guangdong Province (2010B060900052 and 2009B030801186), the Fundamental Research Funds for the Central Universities (the Young Teacher Training Project of Sun Yat-sen University 09ykpy12) and the Medical Scientific Research Project of Zhuhai City (2012003).
Disclosure: The authors declare no conflict of interest.
References
- Nair CK, Parida DK, Nomura T. Radioprotectors in radiotherapy. J Radiat Res 2001;42:21-37. [PubMed]
- Ringborg U, Bergqvist D, Brorsson B, et al. The Swedish Council on Technology Assessment in Health Care (SBU) systematic overview of radiotherapy for cancer including a prospective survey of radiotherapy practice in Sweden 2001—summary and conclusions. Acta Oncol 2003;42:357-65. [PubMed]
- Citrin D, Cotrim AP, Hyodo F, et al. Radioprotectors and mitigators of radiation-induced normal tissue injury. Oncologist 2010;15:360-71. [PubMed]
- Brown DQ, Pittock JW 3rd, Rubinstein JS. Early results of the screening program for radioprotectors. Int J Radiat Oncol Biol Phys 1982;8:565-70. [PubMed]
- Cassatt DR, Fazenbaker CA, Bachy CM, et al. Preclinical modeling of improved amifostine (ethyol) use in radiation therapy. Semin Radiat Oncol 2002;12:97-102. [PubMed]
- Koukourakis MI, Kyrias G, Kakolyris S, et al. Subcutaneous administration of amifostine during fractionated radiotherapy: a randomized phase II study. J Clin Oncol 2000;18:2226-33. [PubMed]
- Rades D, Fehlauer F, Bajrovic A, et al. Serious adverse effects of amifostine during radiotherapy in head and neck cancer patients. Radiother Oncol 2004;70:261-4. [PubMed]
- Waselenko JK, MacVittie TJ, Blakely WF, et al. Medical management strategic national stockpile radiation working group. Ann Intern Med 2004;140:1037-51. [PubMed]
- Gandhi NM, Nair CKK. Radiation protection by diethyldithiocarbamate: protection of membrane and DNA in vitro and in vivo against γ-radiation. J Radiat Res 2004;45:175-80. [PubMed]
- Delanian S, Lefaix JL. The radiation-induced fibroatrophic process: therapeutic perspective via the antioxidant pathway. Radiother Oncol 2004;73:119-31. [PubMed]
- Martin M, Lefaix JL, Crechet F, et al. Temporal modulation of TGF-b1 and b-actin gene expression in pig skin and muscular fibrosis after ionizing radiation. Radiat Res 1993;134:63-70. [PubMed]
- Vozenin MC, Lefaix JL, Ridi R, et al. The myofibroblast markers a-SM actin and b-actin are differentially expressed in 2 and 3-D culture models of fibrotic and normal skin. Cytotechnology 1998;26:29-38. [PubMed]
- Gabbiani G. Modulation of fibroblastic cytoskeletal features during wound healing and fibrosis. Pathol Res Pract 1994;190:851-3. [PubMed]
- Powell DW, Mifflin RC, Valentich JD, et al. Myofibroblasts. Paracrine cells important in health and disease. Am J Physiol 1999;277:C1-9. [PubMed]
- Delanian S, Martin M, Bravard A, et al. Abnormal phenotype of cultured fibroblasts in human skin with chronic radiotherapy damage. Radiother Oncol 1998;47:255-61. [PubMed]
- Lyons A, Ghazali N. Osteoradionecrosis of the jaws: current understanding of its pathophysiology and treatment. Br J Oral Maxillofac Surg 2008;46:653-60. [PubMed]
- Dambrain R. The pathogenesis of osteoradionecrosis. Rev Stomatol Chir Maxillofac 1993;94:140-7. [PubMed]
- Maisin JR. Bacq and Alexander award lecture chemical radioprotection: past, present and future prospects. Int J Radiat Biol 1998;73:443-50. [PubMed]
- Nair CKK, Parida DK, Nomura T. Radioprotectors in Radiotherapy. J Radiat Res 2001;42:21-37. [PubMed]
- Hosseinimehr SJ. Trends in the development of radioprotective agents. Drug Discov Today 2007;12:794-805. [PubMed]
- Kushner I. The phenomenon of the acute phase response. Ann N Y Acad Sci 1982;389:39-48. [PubMed]
- Sottrup-Jensen L. Alpha-macroglobulins: structure, shape, and mechanism of proteinase complex formation. J Biol Chem 1989;264:11539-42. [PubMed]
- Sottrup-Jensen L. a2-macroglobulin and related thiol ester plasma proteins. In: Putnam F. eds. The Plasma Proteins. New York: Academic, 1987:191-291.
- Matthijs G, Devriendt K, Cassiman JJ, et al. Structure of the human alphas-macroglobulin gene and its promoter. Biochem Biophys Res Commun 1992;184:596-603. [PubMed]
- Shi DL, Savona C, Gagnon J, et al. Transforming growth factor-3 stimulates the expression of cs2-macroglobuhin by cultured bovine adrenocortical cells. J Biol Chem 1990;265:2881-7. [PubMed]
- Strauss S, Bauer J, Ganter U, et al. Detection of interleukin-6 and as-macroglobulin immunoreactivity in cortex and hippocampus of Alzheimer’s disease patients. Lab Invest 1992;66:223-30. [PubMed]
- Harpel PC. Human as-macroglobulin. In: Lorand L. eds. Methods in Enzymotogy. New York: Academic, 1976:639-52.
- Goldenberg RL, Tamura T, Cliver SP, et al. Maternal serum alpha-macroglobulin and fetal growth retardation. Obstet Gynecol 1991;78:594-9. [PubMed]
- Ganrot PO, Schersten B. Serum a,-macroglobulin concentration and its variation with age and sex. Clin Cfflm Acta 1967;15:113-20.
- Borth W. α2-Macroglobulin, a multifunctional binding protein with targeting characteristics. FASEB J 1992;6:3345-53. [PubMed]
- Acosta JA, Hoyt DB, Schmid-Scho¨nbein GW, et al. Intraluminal pankreatic serine protease activity, mucosal permeability, and shock: a review. Shock 2006;26:3-9. [PubMed]
- LaMarre J, Wollenberg GK, Gonias SL, et al. Biology of disease: cytokine binding and clearance properties of proteinase-activated a2-macroglobulins. Lab Invest 1991;65:3-14. [PubMed]
- Kratzsch J, Selisko T, Birkenmeier G. Identification of transformed a2-macroglobulin as a growth hormone binding protein in human blood. J Clin Endocrinol Metab 1995;80:585. [PubMed]
- Moestrup SK, Gliemann J, Pallesen G. Distribution of the alpha 2-macroglobulin receptor/low density lipoprotein receptor-related protein in human tissues. Cell Tissue Res 1992;269:375-82. [PubMed]
- Herz J, Hamann U, Rogue S, et al. Surface location and high affinity for calcium of a 500-kDa liver membrane protein closely related to the LDL-receptor suggest a physiological role as lipoprotein receptor. EMBO J 1988;7:4119-27. [PubMed]
- Strickland DK, Ashcom JD, Williams S, et al. Sequence identity between the a2-macroglobulin receptor and low density lipoprotein receptor-related protein suggests that this molecule is a multifunctional receptor. J Biol Chem 1990;265:17401. [PubMed]
- Strickland DK, Kounnas MZ, Argraves WS. LDL receptor-related protein: a multiligand receptor for lipoprotein and proteinase catabolism. FASEB J 1995;9:890. [PubMed]
- Hanna MG, Nettesheim JRP, Fisher WD, et al. Serum alpha globulin fraction: survival-and-recovery effects in irradiated mice. Science 1967;157:1458-61. [PubMed]
- Berenblum I, Burger M, Knyszinski A. Regeneration of bone marrow cells and thymus induced by 19S alpha-2 globulin in irradiated mice. Nature 1968;217:857-8. [PubMed]
- Cotran RS, Remensnyder JP. The structural basis of increased vascular permeability after graded thermal injury-light and electron microscopic studies. Ann NY Acad Sci 1968;150:495-509. [PubMed]
- van Vugt H, van Gool J, de Ridder L. a2-Macroglobulin of the rat, an acute phase protein, mitigate the early course of endotoxin shock. Br J Exp Pathol 1986;67:313-19. [PubMed]
- Ufkes JGR, Ottenhof M, Labruyere WT, et al. The effects of aM-foetoprotein, an acute phase protein, and BaSO4-induced injury on IgE-mediated systemic anaphylaxis in the rat. Br J Exp Pathol 1986;67:321-27. [PubMed]
- Sevaljević L, Petrović M, Bogojević D. Pretreatment with a2-macroglobulin leads to recovery of rats exposed to a lethal scald. Burns 1994;20:122-7. [PubMed]
- Sevaljević L, Dobrić S, Bogojević D, et al. The radioprotective activities of turpentine-induced inflammation and a2-macroglobulin: the effect of dexamethasone on the radioprotective efficacy of the inflammation. J Radiat Res 2003;44:59-67. [PubMed]
- Mihailović M, Dobrić S, Poznanović G, et al. The acute phase protein a2-macroglobulin plays an important role in radioprotection in the rat. Shock 2009;31:607-14. [PubMed]
- Mihailović M, Milosević V, Grigorov I, et al. The radioprotective effect of a2-macroglobulin: a morphological study of rat liver. Med Sci Monit 2009;15:BR188-93. [PubMed]
- Bogojević D, Poznanović G, Grdović N, et al. Administration of rat acute-phase protein a2-macroglobulin before total-body irradiation initiates cytoprotective mechanisms in the liver. Radiat Environ Biophys 2011;50:167-79. [PubMed]
- Riley PA. Free radicals in biology: oxidative stress and the effects of ionizing radiation. Int J Radiat Biol 1994;65:27-33. [PubMed]
- Fridovich I. Superoxide radical and superoxide dismutases. Annu Rev Biochem 1995;64:97-112. [PubMed]
- Murley JS, Kataoka Y, Weydert C, et al. Delayed radioprotection by nuclear transcription factor jBmediated induction of manganese superoxide dismutase in human microvascular endothelial cells after exposure to the free radical scavenger WR1065. Free Rad Biol Med 2006;40:1004-16. [PubMed]
- Matsuda T, Hirano T, Nagasawa S, et al. Identification of alpha 2-macroglobulin as a carrier protein for IL-6. J Immunol 1989;142:148-52. [PubMed]
- Daosukho C, Kiningham K, Kasarskis EJ, et al. Tamoxifen enhancement of TNF-a induced MnSOD expression: modulation of NFκB dimerization. Oncogene 2002;21:3603-10. [PubMed]
- Epperly MW, Defilippi S, Sikora C, et al. Intratracheal injection of manganese superoxide dismutase (MnSOD) plasmid/liposomes protects normal lung but not orthopedic tumors from irradiation. Gene Ther 2000;7:1011-8. [PubMed]
- Vujaskovic Z, Batinic-Haberle I, Rabbani ZN, et al. A small molecular weight catalytic metalloporophyrin antioxidant with superoxide dismutase (SOD) mimetic properties protects lung from radiation-induced injury. Free Radic Biol Med 2002;33:857-63. [PubMed]
- James K. Interactions between cytokines and alpha 2-macroglobulin. Immunol Today 1990;11:163-6. [PubMed]
- Wu SM, Patel DD, Pizzo SV. Oxidized α2-macroglobulin (α2M) differentially regulates receptor binding by citokines/growth factors: implications for tissue injury and repair mechanism in inflammation. J Immunol 1998;161:4356-65. [PubMed]
- Anscher MS, Chen L, Rabbani Z, et al. Recent progress in defining mechanisms and potential targets for prevention of normal tissue injury after radiation therapy. Int J Radiat Oncol Biol Phys 2005;62:255-9. [PubMed]
- Borth W. Alpha 2-macroglobulin. A multifunctional binding and targeting protein with possible roles in immunity and autoimmunity. Ann N Y Acad Sci 1994;737:267-72. [PubMed]
- Kähäri VM, Saarialho-Kere U. Matrix metalloproteinases and their inhibitors in tumour growth and invasion. Ann Med 1999;31:34-45. [PubMed]
- Gonias SL, LaMarre J, Crookston KP, et al. Alpha 2-macroglobulin and the alpha 2-macroglobulin receptor/LRP. A growth regulatory axis. Ann N Y Acad Sci 1994;737:273-90. [PubMed]
- Dinarello CA. Reduction of inflammation by decreasing production of interleukin-1 or by specific receptor antagonism. Int J Tissue React 1992;14:65-75. [PubMed]
- Horn F, Wegenka UM, Lutticken C, et al. Regulation of alpha 2-macroglobulin gene expression by interleukin-6. Ann N Y Acad Sci 1994;737:308-23. [PubMed]
- Bartosz G, Scho¨n W, Kraft G, et al. Irradiation increases proteolysis in erythrocyte ghosts: a spin label study. Radiat Environ Biophys 1992;31:117-21. [PubMed]
- Mainous MR, Erfel W, Chaudry IH, et al. The gut: a cytokine-generating organ in systemic inflammation? Shock 1995;4:193-9. [PubMed]
- Carney JM, Carney AM. Role of protein oxidation in aging and in age-associated neurodegenerative diseases. Life Sci 1994;55:2097-103. [PubMed]
- Mihailović M, Dinic S, Bogojevic D, et al. Nuclear localization and binding affinity of STAT5b for the alpha2-macroglobulin gene promoter during rat liver development and the acute-phase response. Acta Biochim Polon 2007;54:331-40. [PubMed]
- Uskoković A, Dinic S, Mihailovic M, et al. STAT3/NFκB interplay in the regulation of alpha2-macroglobulin gene expression during rat liver development and the acute phase response. IUBMB Life 2007;59:170-8. [PubMed]
- Trocha PJ, Catravas GN. Effect of radioprotectant WR 2721 on cyclic nucleotides, prostaglandins, and lysosomes. Radiat Res 1983;94:239-51. [PubMed]
- Mihajlović M, Vidaković M, Grdović N, et al. The radioprotective efficacy of the rat acute-phase potein alpha2-macroglobulin on bone marrow cells. Genetika 2009;41:29-39.
- Gutterman JU. Cytokine therapeutics: Lessons from inetrferon-α. Proc Natl Acad Sci USA 1994;91:1198-205. [PubMed]
- Neta R, Oppenheim JJ, Schreiber RD, et al. Role of cytokines (inetleukin 1, tumor necrosis factor and transforming growth factor β) in natural and lipopolysaccharide-enhanced radioresistance. J Exp Med 1991;173:1177-82. [PubMed]
- Neta R, Perlstein R, Vogel SN, et al. Role of interleukin 6 (IL-6) in protection from lethal irradiation and in endocrine response to IL-1 and tumor necrosis factor. J Exp Med 1992;175:689-94. [PubMed]
- Ikari Y, Mulvihill E, Schwartz SM. Alpha 1-Proteinase inhibitor, alpha 1-antichymotrypsin, and alpha 2-macroglobulin are the antiapoptotic factors of vascular smooth muscle cells. J Biol Chem 2001;276:11798-803. [PubMed]