Experimental study on enhancement of the metastatic potential of portal vein tumor thrombus-originated hepatocellular carcinoma cells using portal vein serum
Introduction
Hepatocellular carcinoma (HCC) is one of the most common clinical malignancies, whose incidence and mortality rates rank fifth and third among all cancers worldwide (1-3). Characteristics of high recurrence and high metastasis result in a poor prognosis of HCC (4). Portal vein tumour thrombus (PVTT) arising from the invasion of HCC cells into the portal vein, is a special type of intrahepatic metastasis of HCC (5). The formation of PVTT has been considered as one of the main reasons for the poor prognosis of HCC (6). The median survival time of HCC patients with PVTT is less than 6 months (7,8). Even for patients who receive active treatment, the 3-year survival rate is less than 20% (9,10). Therefore, further understanding of these mechanisms is crucial for the development of novel therapeutic strategies and would thereby improve the prognoses of HCC patients.
The metastasis of HCC shows a tendency toward occurring at the portal vein, but rarely at splenic vein and hepatic vein (11). And the incidence of PVTT in HCC patients is high. For example, autopsy and radiographic examination showed that the incidence of HCC PVTT is approximately 20-70%, while microscopic examination revealed an incidence of up to 90% (12). The specificity of metastases to portal vein and high incidence of PVTT may imply the portal vein system may provide a suitable microenvironment for the survival of liver cancer cells, thus allowing induction of the formation and development of PVTT.
In this study, sera were obtained from portal vein blood and peripheral blood collected from HCC patients to mimic tumor microenvironment, and the impact of the different types of sera on the proliferation and metastasis of the PVTT-originated HCC cell line CSQT-2 was observed. The results showed that apoptosis was significantly reduced in the CSQT-2 cells cultured with portal vein serum, while the metastasis and invasiveness of the cells were significantly enhanced. Further investigation showed that compared with peripheral serum, the level of IL-12 in portal vein serum was significantly decreased. Therefore, the portal vein serum could cause the portal vein metastasis of HCC, and this effect may be related to the level of certain cytokines in the serum.
Materials and methods
Patients and collection and preparation of serum
Portal vein blood and peripheral blood were collected from 20 HCC patients receiving treatment in the Eastern Hepatobiliary Surgery Hospital of the Second Military Medical University between 2012 and 2013 with informed consent. All of 20 patients met the following criteria were included: (I) no previous treatment for HCC; (II) no other malignancies; (III) HCC were confirmed by pathological examination. The collected blood samples were stored in a 4 °C refrigerator for 1 h, followed by low-speed centrifugation for 5 min, and the serum in the upper layer was then collected. After filtration through a 0.2 µm sterile filter, the serum to be used for cell culture was treated at a constant temperature of 56 °C for 30 min to inactivate complement proteins. This study was approved by the Ethic Committee of Eastern Hepatobiliary Surgery Hospital.
Cell culture
The PVTT-originated HCC cell line CSQT-2 was established and preserved by our laboratory (13). The cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS, Hyclone, Logan, UT, USA), 100 U/mL penicillin, and 100 µg/mL streptomycin and were maintained in an incubator at 37 °C under 5% CO2. The medium was replaced every 2-3 d, and a passage with 0.25% trypsin digestion was performed when the cells reached 80% confluence. The cells in logarithmic growth phase were selected for culture with DMEM containing 10% portal vein serum or peripheral serum. After culturing for 24 h, the cells were harvested for subsequent experiments.
Cell proliferation assay
Cell proliferation was detected through proliferating cell nuclear antigen (PCNA) fluorescent labeling. Briefly, 1×106 cells from each group were harvested and fixed with cell fixative at room temperature for 30 min, followed by membrane rupture treatment with permeabilization solution for 30 min. The cells were then resuspended, and 2 µL of a PCNA fluorescent antibody was added, followed by incubation in the dark at room temperature for 1 h. Next, 200 µL of propidium iodide (PI) staining solution was added, and the cell samples were incubated at room temperature for 15 min, then washed with PBS and resuspended. Detection was conducted using a flow cytometer (BD Biosciences, San Jose, CA, USA).
Detection of cell cycle stages and apoptosis
The cells to be used for the detection of cell cycle stages were trypsinized and then fixed with pre-chilled 70% ethanol overnight. A 50 µg/mL PI staining solution containing RNase was added, followed by incubation at 37 °C for 30 min. The proportions of the cells at each phase were detected using a flow cytometer.
Cell apoptosis was detected using an Annexin V-FITC/PI apoptosis detection kit (KeyGen Biotech, Nanjing, China). The cells were harvested via trypsinization when they reached 80% confluence, then washed with PBS and resuspended with 500 µL of Annexin V-binding buffer. Subsequently, 5 µL of FITC-labeled Annexin V and 5 µL of PI staining solution were added. Flow cytometry detection was performed after incubation at room temperature in the dark for 15 min.
Detection of cell migration and invasion
Cell migration and invasion were detected using Transwell chambers (Corning Incorporated Life Sciences, Tewksbury, MA, USA). For the migration assay, 1×104 cells were directly seeded in the upper Transwell chamber. For the invasion assay, the upper chamber was coated with Matrigel (BD Biosciences, San Jose, CA, USA) before cell seeding. In the lower chamber, 800 µL of DMEM containing 10% fetal bovine serum was added as culture medium, and the cells were cultured in the cell incubator. After culturing for 24 h, the medium was discarded. The cells were fixed with 100% methanol for 10 min and then re-fixed with 75% methanol-acetic acid for another 10 min. After incubation with 0.2% Triton-X-100 for 5 min, the un-penetrated cells in the upper chamber were wiped with a swab and stained with 0.1% DAPI for 5 min. Under a fluorescence microscope, five fields (upper left, lower left, upper right, lower right, and center) were selected to count the number of nuclei that passed through the small chamber.
Western blotting
Huh7 cells were cultured and manipulated using the same methods, and the cells in each group were harvested after 24 h. The cells were lysed with RIPA lysis buffer (Beyotime Institute of Biotechnology, Haimen, China), and the protein concentration was determined through the BCA method and then normalized. For each group, 40 µg of total protein was loaded for SDS-PAGE, and following electrophoresis, the proteins were electrotransferred to a PVDF membrane (Millipore, Bedford, MA, USA). After blocking with 5% skim milk at room temperature for 1 h, the diluted primary antibody (dilution of 1:200 for both MMP-2 and MMP-9, Wanleibio, Shenyang, China) was added, followed by incubation at 4 ˚C overnight. A horseradish peroxidase (HRP)-conjugated secondary antibody (Beyotime Institute of Biotechnology) at a 1:5,000 dilution was added, followed by incubation at 37 ˚C for 1 h. Substrate luminescence was determined using the ECL method. After exposure, the obtained image was scanned into a computer.
Detection of cytokines
The contents of IL-12, IFN-γ, IL-1β, IL-2 and TNF-α in the two types of serum were detected using the FlowCytomix Human Th1/Th2/Th9/Th17/Th22 13 plex kit (eBioscience, San Diego, CA, USA). The experimental operation strictly followed the instructions of the kit. Data processing and analysis were conducted using FlowCytomix Pro3.0 software.
Statistical analysis of the data
The experimental data are represented as the mean ± standard deviation. Comparisons between two groups were performed using one-way analysis of variance, while comparisons among multiple groups were carried out with the Bonferroni post hoc test. The data were processed using GraphPad Prism 5.0 software, and P<0.05 was considered to indicate a statistically significant difference.
Results
Portal vein blood could inhibit the apoptosis of CSQT-2 cells
To investigate the impact of the different types of sera on the proliferation of CSQT-2 cells, cell proliferation and cell cycle phases were detected via flow cytometry (Figure 1A,B). The results showed that cell proliferation and cell cycle stages did not differ significantly between the cells cultured in portal vein serum and peripheral serum (P>0.05), and the different types of sera therefore did not affect the proliferation of HCC PVTT cells. The impact of the two types of sera on the apoptosis of CSQT-2 cells was further analyzed via flow cytometry (Figure 1C), and the results showed a significant reduction in the proportion of apoptotic cells cultured in portal vein serum (P<0.01). Collectively, these results suggest portal vein blood may have an inhibitory effect on the apoptosis of cancer cells.
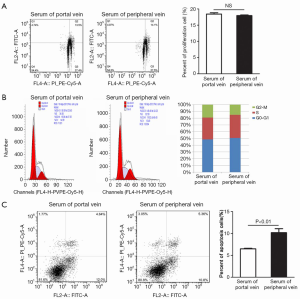
Portal vein blood could promote the migration and invasion of CSQT-2 cells
To ascertain whether portal vein blood can promote the metastasis of cancer cells, a Transwell chamber was used to detect the impact of the two types of sera on the migration and invasion of CSQT-2 cells. Under a fluorescence microscope, the number of migrating cells in the portal serum group was found to be significantly higher than that in the peripheral serum group in the migration assay (Figure 2A, P<0.01). Furthermore, the two groups of cells were seeded into the small chamber coated with Matrigel to observe the invasiveness of the cells (Figure 2B). The result of this assay was consistent with that of the migration assay. The number of invaded cells in the portal vein serum group was significantly higher than that in the peripheral serum group (P<0.01). Therefore, all of these results indicate portal vein blood could increase the migration and invasion of cancer cells.
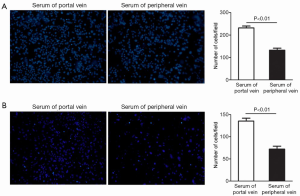
Portal vein blood could up-regulate the expression of matrix metalloproteinase-2 (MMP-2)
The matrix metalloproteinase family (MMPs) that are considered to be strongly associated with tumor metastasis, we next to determined whether MMPs were involved in the portal vein serum-mediate metastases. CSQT-2 and human Huh7 HCC cells were treated with the two types of sera, and the expression of MMP-2 and MMP-9 was subsequently detected via Western blotting (Figure 3). The results showed that the expression of MMP-2 in both cells cultured in portal vein serum were significantly enhanced, indicating that portal vein blood promote PVTT invasion by up-regulating MMP-2 expression.
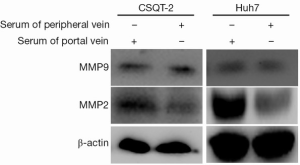
The effects of portal vein blood on the induction of metastasis and inhibition of apoptosis in cancer cells are related to a reduced level of IL-12
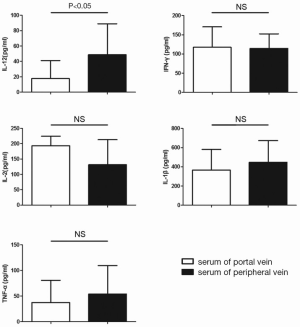
To further investigate the mechanism underlying the effects of portal vein blood in the induction of metastasis and inhibition of apoptosis, the levels of IL-12, IFN-γ, IL-2, IL-2β and TNF-α in the two types of sera were tested (Figure 4). It was found that the level of IL-12 in portal vein serum was significantly lower than that in peripheral serum (P<0.05), while the contents of other cytokines were not significantly different (P>0.05). Therefore, the effects of portal vein blood concerning metastasis induction and apoptosis inhibition in patients with HCC may be related to the reduced level of IL-12.
Discussion
The tumor microenvironment is closely related to tumor development, which affects the occurrence, metastasis and disappearance of tumors (14). The metastasis of HCC shows a tendency toward occurring at the portal vein, which may be closely related to the special microenvironment of the portal venous system. In this study, portal vein serum and peripheral serum were used to mimic tumor microenvironment and the cell behaviors had been observed. The results showed that compared with peripheral serum, portal vein serum was able to inhibit the apoptosis and enhance the migration and invasion capacities of the cancer cells. These results suggest portal venous system is suitable for survival of tumor cells and further metastases, which confirmed our hypothesis and further clarified the pathogenesis of PVTT.
Most of HCC arise in the setting of chronic hepatitis induced by hepatitis virus infection (hepatitis B virus, HBV or hepatitis C virus, HCV). The virus infection-triggered inflammatory and/or fibrotic process, with extensive involvement of cytokine/chemokine production/activation and leukocytes infiltration, is believed to create a microenvironment that favors the development of HCC (15). For example, cytokines including TGF-β, TNF-α, IL-6, IL-17 and IL-10 involved in the pathogenesis of HCC, promoting proliferation, invasion, angiogenesis and metastasis. More intriguingly, HBV-infection status and cytokines also have been found to associate with PVTT. Yang et al. showed that a link between the development of PVTT and positive HBV infection status, as well as high level of TGF-β signaling activity (16). Increased TGF-β in portal venous system enhanced production of the chemokine CCL22 and recruitment of Treg cells, which leading to the development of intrahepatic venous metastasis. However, the component of PVTT microenvironment is complex and of which responsible for PVTT formation or development need to be further explored.
In the present study, we also examined the levels of cytokines (IL-12, IFN-γ, IL-2, IL-1β and TNF-α) in the portal vein serum and the peripheral serum. The results showed that, compared with peripheral blood, the level of IL-12 in portal vein serum was significantly lower, while no significant differences were observed for the other cytokines. It is now clear that the IL-12 family is a class of tumor immune factors that can be involved in cell immunity themselves or in the induction of other cytokines, and the members of this family have a synergistic anti-tumor effect with NK cells and T lymphocytes (17-19). Moreover, Yin et al. showed that IL-12 exhibit a direct effect for reducing invasiveness and increasing apoptosis in colon cancer stem cells (20). Zhou et al. showed that IL-12 up-regulates Fas expression which increasing their sensitivity to Fas-induced cell apoptosis (21). Based on these findings, we considered that the reduced level of IL-12 in portal vein serum may result in the relatively weak ability of the portal vein system to resist tumor cells, thus readily leading to cancer metastasis.
Moreover, we also found the expression of MMP-2 was significantly up-regulated in tumor cells treated with portal vein serum. As an important member of the matrix metalloproteinase family, MMP-2 not only plays an important role in tumor metastasis but also serves as one of the key indicators determining the metastasis of tumor infiltration agents (22,23). Although IL-12 has been found to up-regulate the expression of MMP-2 in monocytic cells (24), there is no direct evidence that IL-12 exhibit the same effect on tumor cells. Therefore, there would be other substances in portal vein blood be responsible for PVTT invasion by up-regulating MMP2. In fact, the mechanism of the formation and development of PVTT is complex. A variety of cytokines, adhesion molecules and miRNAs in the portal vein system are involved in the formation and development of PVTT (25-28). Moreover, an abundant of substance, including cytokines, immune cells, protein and mRNA in portal vein blood, whether these substances would contribute to the formation and development of PVTT cannot be excluded.
In summary, the present study demonstrated that portal vein blood can promote the metastasis and invasion of PVTT-originated tumor cells, and this effect may be related to the reduced level of IL-12. This finding lays a foundation for further elucidation of the mechanism of portal vein metastasis. In a subsequent study, we will further examine the effects of tumor immune factors on PVTT-originated tumor cells to clarify the role of IL-12 and other tumor immune factors in the formation and development of PVTT.
Conclusions
Portal vein serum from HCC patients could inhibit the apoptosis of PVTT-originated HCC cells and promote cell metastasis and invasion. These effects may be related to the lower level of IL-12 in portal vein serum. All of these results suggest microenvironment of portal venous system is suitable for survival of tumor cells and further metastases.
Acknowledgements
Grant Support: This work was supported by the China National Funds for the Science Fund for Creative Research Groups (No: 81221061); The State Key Project on Infections Diseases of China (2012zx10002016016003); China National Funds for the Distinguished Young Scientists (No: 81125018); National Natural Science Foundation(No: 81071750, 81101831, 81101511); New Excellent Talents Program of Shanghai Municipal Health Bureau (No: XBR2011025); Shanghai Science and Technology Committee (No. 134119a0200;10JC1417600); Program for New Century Excellent Talents in University and specially-appointed Professor of Shanghai; SMMU Innovation Alliance for Liver Cancer Diagnosis and Treatment [2012]. Chang Jiang Scholars Program [2012].
Disclosure: The authors declare no conflict of interest.
References
- Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer 2006;118:3030-44. [PubMed]
- Portolani N, Coniglio A, Ghidoni S, et al. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann Surg 2006;243:229-35. [PubMed]
- Yamane B, Weber S. Liver-directed treatment modalities for primary and secondary hepatic tumors. Surg Clin North Am 2009;89:97-113. [PubMed]
- Kornek M, Raskopf E, Tolba R, et al. Accelerated orthotopic hepatocellular carcinomas growth is linked to increased expression of pro-angiogenic and prometastatic factors in murine liver fibrosis. Liver Int 2008;28:509-18. [PubMed]
- Kaibori M, Ishizaki M, Matsui K, et al. Predictors of microvascular invasion before hepatectomy for hepatocellular carcinoma. J Surg Oncol 2010;102:462-8. [PubMed]
- Shuqun C, Mengchao W, Han C, et al. Tumor thrombus types influence the prognosis of hepatocellular carcinoma with the tumor thrombi in the portal vein. Hepatogastroenterology 2007;54:499-502. [PubMed]
- Li N, Guo W, Shi J, et al. Expression of the chemokine receptor CXCR4 in human hepatocellular carcinoma and its role in portal vein tumor thrombus. J Exp Clin Cancer Res 2010;29:156. [PubMed]
- Villa E, Moles A, Ferretti I, et al. Natural history of inoperable hepatocellular carcinoma: estrogen receptors’ status in the tumor is the strongest prognostic factor for survival. Hepatology 2000;32:233-8. [PubMed]
- Shi J, Lai EC, Li N, et al. Surgical treatment of hepatocellular carcinoma with portal vein tumor thrombus. Ann Surg Oncol 2010;17:2073-80. [PubMed]
- Chen JS, Wang Q, Chen XL, et al. Clinicopathologic characteristics and surgical outcomes of hepatocellular carcinoma with portal vein tumor thrombosis. J Surg Res 2012;175:243-50. [PubMed]
- Webster GJ, Burroughs AK, Riordan SM. Review article: portal vein thrombosis—new insights into aetiology and management. Aliment Pharmacol Ther 2005;21:1-9. [PubMed]
- Cedrone A, Rapaccini GL, Pompili M, et al. Portal vein thrombosis complicating hepatocellular carcinoma. Value of ultrasound-guided fine-needle biopsy of the thrombus in the therapeutic management. Liver 1996;16:94-8. [PubMed]
- Wang T, Hu HS, Feng YX, et al. Characterisation of a novel cell line (CSQT-2) with high metastatic activity derived from portal vein tumour thrombus of hepatocellular carcinoma. Br J Cancer 2010;102:1618-26. [PubMed]
- Lorusso G, Rüegg C. The tumor microenvironment and its contribution to tumor evolution toward metastasis. Histochem Cell Biol 2008;130:1091-103. [PubMed]
- Hernandez-Gea V, Toffanin S, Friedman SL, et al. Role of the microenvironment in the pathogenesis and treatment of hepatocellular carcinoma. Gastroenterology 2013;144:512-27. [PubMed]
- Yang P, Li QJ, Feng Y, et al. TGF-β-miR-34a-CCL22 signaling-induced Treg cell recruitment promotes venous metastases of HBV-positive hepatocellular carcinoma. Cancer Cell 2012;22:291-303. [PubMed]
- Suzuki R, Namai E, Oda Y, et al. Cancer gene therapy by IL-12 gene delivery using liposomal bubbles and tumoral ultrasound exposure. J Control Release 2010;142:245-50. [PubMed]
- Dowell AC, Oldham KA, Bhatt RI, et al. Long-term proliferation of functional human NK cells, with conversion of CD56(dim) NK cells to a CD56 (bright) phenotype, induced by carcinoma cells co-expressing 4-1BBL and IL-12. Cancer Immunol Immunother 2012;61:615-28. [PubMed]
- Weiss JM, Subleski JJ, Wigginton JM, et al. Immunotherapy of cancer by IL-12-based cytokine combinations. Expert Opin Biol Ther 2007;7:1705-21. [PubMed]
- Yin XL, Wang N, Wei X, et al. Interleukin-12 inhibits the survival of human colon cancer stem cells in vitro and their tumor initiating capacity in mice. Cancer Lett 2012;322:92-7. [PubMed]
- Zhou Z, Lafleur EA, Koshkina NV, et al. Interleukin-12 up-regulates Fas expression in human osteosarcoma and Ewing’s sarcoma cells by enhancing its promoter activity. Mol Cancer Res 2005;3:685-91. [PubMed]
- Heissig B, Hattori K, Friedrich M, et al. Angiogenesis: vascular remodeling of the extracellular matrix involves metalloproteinases. Curr Opin Hematol 2003;10:136-41. [PubMed]
- Giannelli G, Bergamini C, Marinosci F, et al. Clinical role of MMP-2/TIMP-2 imbalance in hepatocellular carcinoma. Int J Cancer 2002;97:425-31. [PubMed]
- Abraham M, Shapiro S, Lahat N, et al. The role of IL-18 and IL-12 in the modulation of matrix metalloproteinases and their tissue inhibitors in monocytic cells. Int Immunol 2002;14:1449-57. [PubMed]
- Pawlik TM, Poon RT, Abdalla EK, et al. Hepatectomy for hepatocellular carcinoma with major portal or hepatic vein invasion: results of a multicenter study. Surgery 2005;137:403-10. [PubMed]
- Zhou J, Tang ZY, Fan J, et al. Expression of platelet-derived endothelial cell growth factor and vascular endothelial growth factor in hepatocellular carcinoma and portal vein tumor thrombus. J Cancer Res Clin Oncol 2000;126:57-61. [PubMed]
- Liu S, Guo W, Shi J, et al. MicroRNA-135a contributes to the development of portal vein tumor thrombus by promoting metastasis in hepatocellular carcinoma. J Hepatol 2012;56:389-96. [PubMed]
- Zhou J, Tang Z, Fan J. Expression rates of three angiogenic factors in hepatocellular carcinoma and their relation with microvessel density and portal vein tumor thrombosis. Zhonghua Yi Xue Za Zhi 2001;81:462-4. [PubMed]


