Evaluation of contrast-enhanced ultrasound for diagnosis of dysplastic nodules with a focus of hepatocellular carcinoma in liver cirrhosis patients
Introduction
Since chronic liver disease is one of the most important factors in carcinogenesis, hepatocellular carcinoma (HCC) often occurs in association with liver cirrhosis (1). Some reports have proposed that the stepwise carcinogenesis of HCC progresses from regenerative nodules (RN), to low-grade dysplastic nodules (DNs) or high-grade DNs, to DNs with a focus of HCC (DN-HCC), and finally to HCC (2-4). To distinguish nodules in patients at high risk for HCC is very important for doctors who perform ultrasonography. Because of the complicated liver texture, it is difficult to differentiate focal liver lesions within a cirrhotic background by conventional ultrasound (US) or even by color Doppler imaging (3,5-9). A contrast-enhanced US (CEUS) can define the microcirculation characteristics in liver nodules, so it is helpful for diagnosis of benign and malignant lesions. In recent years, some clinical research and applications of various kinds of contrast agents and US signal detection techniques have been focused on the evaluation of liver tumors, but very few studies have focused on the enhancement patterns of DN-HCC (10-14). In the present study, the characteristics of DN-HCC in cirrhotic livers were observed on CEUS by using SonoVue® (Bracco SpA, Milan, Italy) to explore the value of CEUS in the diagnosis of DN-HCC and the guidance of the biopsy.
Material and methods
Materials
During the time period from March 2004 to December 2013, 193 cirrhotic patients with 215 focal liver lesions, ranging in size from 1.0 to 3.5 cm on conventional US, were enrolled in our study. There were 141 males and 52 females, and their ages ranged from 32 to 77 years. All patients underwent biopsy immediately after CEUS. The biopsies were performed in the different areas of enhancement.
This study received institutional review board approval of Beijing Cancer Hospital, and patients gave informed consent.
Contrast agent and ultrasound (US) procedures
The US contrast agent, SonoVue, was administered intravenously as 1.5-2.4 mL boluses through the antecubital vein within 2-3 s, immediately followed by flushing with 5 mL 0.9% saline. CEUS was performed using the Technos DU8 US system (Esaote, Milan, Italy), GE LOGIQ E9 or 9 (Milwaukee, WI, USA) or Philips IU-22 (Bothell, WA, USA) with probe frequencies of 2.5 to 5.0 MHz.
The mechanical index (MI) was adjusted to about 0.06-0.10 based on the lesion depth. The perfusion and enhancement patterns of the target lesions were continuously observed throughout all phases of CEUS for about 4-6 min. To obtain all phases of the lesions, the probe remained fixed and respiration was controlled. If the first injection was not satisfactory or additional fields needed to be observed, a second contrast injection was given 10-15 min later. The CEUS diagnosis of the lesions was made based on the enhancement patterns during the arterial phase (0-30 s), the portal phase (31-120 s), and the late phase (after 120 s).
Imaging analysis
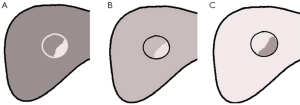
The surrounding liver parenchyma was used as a control. The enhancement of the lesion earlier than, later than, or equal to the surrounding parenchyma was defined as early enhancement, late enhancement, or equal enhancement, respectively. The washout was conducted similarly. Lesion enhancement greater than, less than, or equal to the surrounding parenchyma was defined as hyper-, hypo-, or iso-enhancement, respectively. If the lesion showed different degrees of enhancement, it was defined as heterogeneous enhancement. The enhancement patterns for all nodules were observed during the arterial phase (0-30 s), the portal phase (31-120 s), and the late phase (>120 s).
Diagnosis methods
All patients underwent biopsy immediately after CEUS. Usually the samples were obtained using 18-gauge needles (Bard, Crawley, UK) in the different enhanced areas. The shot length was set up to 1.5 or 2.2 cm depending on the nodule size. The number of puncture attempts was decided by the quantity of the specimen obtained. Repeat CEUS examinations were performed every 3-6 months for patients with negative biopsies. If a part of a nodule presented abnormal enhancement in the follow-up examination, a repeat biopsy was needed.
The pathological examination was performed by two pathologists with more than 20 years of experience. HCC and RN were diagnosed using standard diagnostic criteria of the International Working Party (1). DN-HCC was diagnosed as a focus of carcinoma in a DN.
Statistical analysis
The SPSS 10.0 software (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. The study data were analyzed with a Chi-squared test. The level of statistical significance was set at P<0.05.
Results
Characteristics of patients
The characteristics of patients are shown in Table 1. There were 86 HCC lesions, 102 RN lesions, and 27 DN-HCC lesions diagnosed by biopsy. Patients with HCC, RN, and DN-HCC underwent two or three puncture attempts.
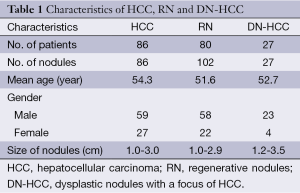
Full table
Enhancement patterns
The appearance of HCC, RN and DN-HCC on CEUS is shown in Table 2 and Figure 1. There were statistically significant differences in the appearance of HCC, RN and DN-HCC on CEUS (P<0.001).
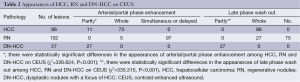
Full table
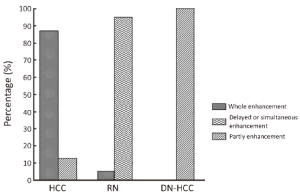
Of 86 HCC lesions, 87.2% (75/86) showed complete enhancement during the arterial phase or the portal phase, and 12.8% (11/86) had inhomogeneous enhancement, with no enhancement in the central area, during the arterial phase; 100% (86/86) exhibited washout during the late phase (Figure 2). Of 102 RN lesions, 95.1% (97/102) showed delayed or simultaneous enhancement during the arterial phase, and 4.9% (5/102) had only slight enhancement during the arterial phase; 26.5% (27/102) showed washout and 73.5% (75/102) showed no washout during the late phase (Figure 3). In 27 DN-HCC lesions, a part of the lesions enhanced during the arterial phase and washed out during the late phase; the other areas exhibited delayed or simultaneous enhancement during the arterial phase and slight washout in the late phase (Figures 4,5).
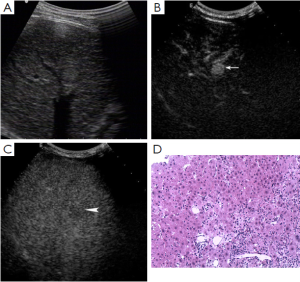
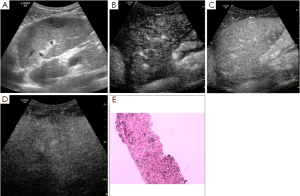
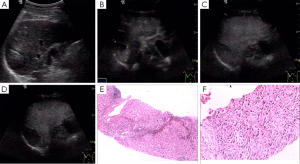
In 86 HCC lesions, the pathological diagnosis was HCC in the enhanced area of 75 lesions, hepatocellular fatty degeneration in the slightly enhanced area of 7 lesions, and hepatocellular necrosis in the unenhanced area and HCC in the enhanced area of 4 lesions. In 102 RN lesions, the pathological diagnosis was hepatocyte proliferation with or without fatty degeneration. In 27 DN-HCC lesions, the pathological diagnosis was HCC in the enhanced area and hepatocyte proliferation in the unenhanced area.
Discussion
Early diagnosis of HCC is important because treatment is most effective when the tumor is small (2,15,16). DNs may develop into carcinoma (4). Early detection of DNs with small areas of HCC is helpful for effective treatment. US is usually used for following hepatic cirrhosis, and it has sensitivity for small nodules with hepatic cirrhosis. However, it is of limited utility for differentiating benign from malignant lesions. For HCC, a stepwise process of carcinogenesis has been proposed, involving changes in the blood supply and perfusion of the nodules (2,17-19). The characteristics of the blood supply in hepatic nodules are the pathophysiological basis of early detection, diagnosis and prognosis of lesions. SonoVue, a blood-pool marker used in CEUS, is helpful for defining the characteristics of the blood supply in hepatic nodules, which play an important role in carcinogenesis and HCC tumor growth. Thus, CEUS is helpful for the early diagnosis of RN and small HCC in a background of hepatic cirrhosis with multiple nodules (10-13). However, very few studies have focused on the enhancement patterns of DN-HCC in cirrhotic patients.
In this study, we focused on the perfusion and echo-change of DN-HCC and examined the value of CEUS in the diagnosis of DN-HCC and biopsy guidance compared with HCC and RN.
Diagnosis and differential diagnosis of DN-HCC in CEUS
The intranodular blood supply of RN is similar to the surrounding parenchyma, within which there is no obviously abnormal blood supply. However, the intranodular portal venous supply gradually decreases, whereas the intranodular arterial supply first decreases and then increases in accordance with an increase in the grade of malignancy of the hepatocellular nodule (1,17-19). As most studies have described, no enhancement during the arterial phase and isoechoic staining in the portal and late phases were detected in RN with CEUS, which was histologically in accordance with no obviously abnormal blood supply in RN (20,21). Recently, many studies have shown that CEUS was helpful for the detection of HCC and the differential diagnosis with RN. In the arterial phase, a hypervascular HCC nodule exhibited enhancement while RN had no enhancement (22-24). Moreover, in the late phase, most HCC nodules exhibited washout while RN was usually similar to the surrounding hepatic parenchyma. Thus, RN or HCC could be diagnosed correctly depending on different enhancement patterns (5,25). DN-HCC nodules are composed of two different cells, high grade differential HCC and atypical hepatic cells. Therefore, different manifestations were found in the same nodule on CEUS. In this study, a part of the 27 lesions of DN-HCC enhanced during the arterial or the portal phase and washed out during the late phase, which was similar to HCC; the other areas exhibited delayed or simultaneous enhancement during the arterial phase and simultaneous or earlier washout during the late phase, which was similar to RN. Therefore, the nodule in a cirrhotic liver might be DN-HCC if it is partly like HCC and partly like RN.
CEUS before biopsy improves diagnostic accuracy
The information yielded by CEUS performed before biopsy procedures using conventional US guidance can significantly decrease the false-negative rate for malignant lesions. CEUS can be used to localize the site for biopsy more accurately by differentiating areas of viable tumor from denaturalization or necrosis, which reduces the number of puncture attempts and significantly increases the success rate of biopsy. In our study, the pathological diagnosis was HCC in the enhanced area and hepatocyte regeneration in the unenhanced area. A biopsy of DN-HCC might give false-negative results without CEUS guidance, which might affect the early detection and treatment of tumors.
Conclusions
It is important to distinguish among RN, HCC and DN-HCC in cirrhosis with small intrahepatic lesions. CEUS can be used to localize the site for biopsy more accurately by differentiating areas of DN-HCC from HCC or RN. CEUS may be used as a routine surveillance tool for cirrhosis patients with small nodules based on its simple performance, minor side effects, and high diagnostic specificity.
Acknowledgements
Funding: This study was supported by the National Natural Science Foundation of China (No. 81471768) and Training Program of the Health Research Plan of the Capital Citizens of Beijing Municipal Science & Technology Commission (No. Z111107067311026).
Disclosure: The authors declare no conflict of interest.
References
- International Working Party. Terminology of nodular hepatocellular lesions. Hepatology 1995;22:983-93. [PubMed]
- Choi BI. Hepatocarcinogenesis in liver cirrhosis: imaging diagnosis. J Korean Med Sci 1998;13:103-16. [PubMed]
- Park YN, Kim MJ. Hepatocarcinogenesis: imaging-pathologic correlation. Abdom Imaging 2011;36:232-43. [PubMed]
- Matsui O, Kobayashi S, Sanada J, et al. Hepatocelluar nodules in liver cirrhosis: hemodynamic evaluation (angiography-assisted CT) with special reference to multi-step hepatocarcinogenesis. Abdom Imaging 2011;36:264-72. [PubMed]
- Quaia E, Degobbis F, Tona G, et al. Differential patterns of contrast enhancement in different focal liver lesions after injection of the microbubble US contrast agent SonoVue. Radiol Med 2004;107:155-65. [PubMed]
- Suzuki Y, Fujimoto Y, Hosoki Y, et al. Clinical utility of sequential imaging of hepatocellular carcinoma by contrast-enhanced power Doppler ultrasonograpy. Eur J Radiol 2003;48:214-9. [PubMed]
- Lim JH, Choi BI. Dysplastic nodules in liver cirrhosis: imaging. Abdom Imaging 2002;27:117-28. [PubMed]
- Tanaka S, Kitamra T, Fujita M, et al. Small hepatocellular carcinoma: differentiation from adenomatous hyperplastic nodule with color Doppler flow imaging. Radiology 1992;182:161-5. [PubMed]
- Lencioni R, Bartolozzi C, Caramella D, et al. Management of adenomatous hyperplastic nodules in the cirrhotic liver: US follow-up or percutaneous alcohol ablation? Abdom Imaging 1993;18:50-5. [PubMed]
- Wu W, Chen MH, Sun M, et al. Contrast-enhanced ultrasound of hepatocarcinogenesis in liver cirrhosis. Chin Med J (Engl) 2012;125:3104-9. [PubMed]
- Kim TK, Jang HJ. Contrast-enhanced ultrasound in the diagnosis of nodules in liver cirrhosis. World J Gastroenterol 2014;20:3590-6. [PubMed]
- Isozaki T, Numata K, Kiba T, et al. Differential diagnosis of hepatic tumors by using contrast enhancement patterns at US. Radiology 2003;229:798-805. [PubMed]
- Numata K, Luo W, Morimoto M, et al. Contrast enhanced ultrasound of hepatocellular carcinoma. World J Radiol 2010;2:68-82. [PubMed]
- Kim TK, Lee KH, Khalili K, et al. Hepatocellular nodules in liver cirrhosis: contrast-enhanced ultrasound. Abdom Imaging 2011;36:244-63. [PubMed]
- Eskesen AN, Bjøro K, Aandahl EM, et al. Low use of surveillance and early diagnosis of hepatocellular carcinoma in Norway--a population-based cohort study. Cancer Epidemiol 2014;38:741-7. [PubMed]
- Chen MH, Yan K, Yang W, et al. Long term (5 years) outcome of radiofrequency ablation for hepatocellular carcinoma in 256 cases. Beijing Da Xue Xue Bao 2005;37:671-2. [PubMed]
- Matsui O, Kadoya M, Kameyama T, et al. Benign and malignant nodules in cirrhotic livers: distinction based on blood supply. Radiology 1991;178:493-7. [PubMed]
- Matsui O, Ueda K, Kobayashi S, et al. Intra- and perinodular hemodynamics of hepatocellular carcinoma: CT observation during intra-arterial contrast injection. Abdom Imaging 2002;27:147-56. [PubMed]
- Hayashi M, Matsui O, Ueda K, et al. Progression to hypervascular hepatocellular carcinoma: correlation with intranodular blood supply evaluated with CT during intraarterial injection of contrast material. Radiology 2002;225:143-9. [PubMed]
- Quaia E, Calliada F, Bertolotto M, et al. Characterization of focal liver lesions with contrast-specific US modes and a sulfur hexafluoride-filled microbubble contrast agent: diagnostic performance and confidence. Radiology 2004;232:420-30. [PubMed]
- Wen YL, Kudo M, Zheng RQ, et al. Characterization of hepatic tumors: value of contrast-enhanced coded phase-inversion harmonic angio. AJR Am J Roentgenol 2004;182:1019-26. [PubMed]
- Loria F, Loria G, Basile S, et al. Contrast-enhanced ultrasound of hepatocellular carcinoma: correlation between enhancement pattern and cellular differentiation on histopathlogy. Updates Surg 2012;64:247-55. [PubMed]
- Kim TK, Choi BI, Han JK, et al. Hepatic tumors: contrast agent-enhancement patterns with pulse-inversion harmonic US. Radiology 2000;216:411-7. [PubMed]
- Wilson SR, Burns PN, Muradali D, et al. Harmonic hepatic US with microbubble contrast agent: initial experience showing improved characterization of hemangioma, hepatocellular carcinoma, and metastasis. Radiology 2000;215:153-61. [PubMed]
- Catalano O, Lobianco R, Cusati B, et al. Hepatocellular carcinoma: spectrum of contrast-enhanced gray-scale harmonic sonography findings. Abdom Imaging 2004;29:341-7. [PubMed]


