Interaction between circulating cancer cells and platelets: clinical implication
Introduction
More than 90% of all cancer-associated deaths are caused by metastasis, a cascade of events beginning with the epithelial-mesenchymal transition (EMT) (1). EMT, as well as its reverse process, mesenchymal-epithelial transition (MET), play pivotal roles in organ development, tissue repair and cancer metastasis by endowing epithelial cells with enhanced migratory capacity, elevated resistance to apoptosis and increased production of ECM components (2). A subpopulation of cancer cells, which undergo an EMT/MET stage, can detach from the primary site, invade through the surrounding tissue, enter and survive in the circulation, and proliferate in a foreign microenvironment (3,4). These cells are called circulating tumor cells (CTCs) (5). The presence of CTCs in patients with carcinoma is associated with a poor prognosis because CTCs may reach a secondary organ prior to the appearance of clinical symptoms. Therefore, CTCs may represent not only a prognostic marker but also be a promising target for anticancer therapies. To exploit the window of opportunity for clinical intervention, a better understanding of the biological behaviors of CTCs is required.
Thrombocytosis is frequently observed in patients with metastatic malignant tumors (6,7). The risk of venous thromboembolism (VTE), including deep venous thrombosis (DVT) and pulmonary embolism (PE), is increased up to seven-fold in these patients compared with non-cancer patients (8-10). These clinical data suggest that platelets may contribute to metastasis, in addition to their well-known role in hemostasis and coagulation. Platelets are an anucleate, discoid shaped blood cell, which contain three types of secretory granules, α-granules, dense granules, and lysosomes (11). Alpha granules, which are the most abundant granules in platelets, include large proteins contributing to adhesion and aggregation. Dense granules contain small, nonprotein substances, which upon secretion, recruit subsequent platelets. Lysosomes primarily secrete hydrolases involved in the elimination of platelet aggregates (7). To further demonstrate the contribution of platelets to metastatic processes, several studies using established animal models reported that platelets promote metastasis by protecting tumor cells from host immune surveillance and enhancing CTCs-endotheliocyte adhesion (12-14). Although the underlying molecular mechanisms have not been completely elucidated, advances in our understanding of metastasis have highlighted the importance of direct or indirect interactions between platelets and CTCs.
Role of platelets in angiogenesis
Angiogenesis is a rate-limiting process in cancer metastasis. The formation of CTCs is hampered by tight vascular wall barriers (15). However, the neovasculature of primary tumors typically has weak and leaky endothelial cell junctions, which facilitates transendothelial migration (TEM) (16-20). Platelets contain angiogenic and angiostatic factors, and the switch to an angiogenesis phenotype can be triggered by metastasizing tumor cells (21,22). Tumor cells can function indirectly via binding to von Willebrand factor (vWF) to initiate platelet aggregation, resulting in the release of vascular endothelial growth factor (VEGF), one of the most powerful positive regulators of angiogenesis (23). Ligation of the protease-activated receptor-1 (PAR-1) can also promote the release of VEGF-containing α-granules (24,25). By contrast, PAR-4 activation up-regulates the secretion of endostatin, a platelet-derived angiogenesis inhibitor (Figure 1) (25).
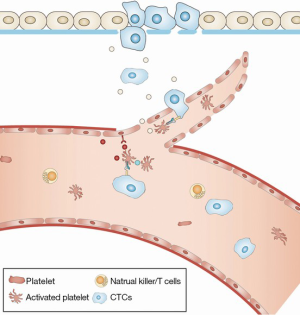
Platelets also affect angiogenesis by seeding microparticles, which express platelet surface antigens, including CD41 and CD42b (26,27). Metastasizing cancer cells can activate platelets at the primary site, increasing the local concentration of platelet microparticles (PMPs). After fusing with target cancer cells, PMPs may deliver pro-angiogenic factors, such as basic fibroblast growth factor (bFGF) and VEGF (28). Additionally, circulating PMPs may up-regulate the level of matrix metalloproteinase 2 (MMP2) in prostate cancer cells and facilitate the intravasation of metastasizing cancer cells (29). The increased invasive potential of tumor cells induced by PMPs further confirms a robust interaction between platelets and CTCs (30).
Interactions between platelets and primary tumor cells
Although the mechanisms by which platelets act are poorly understood, there is evidence that primary tumor cells express thrombin to promote metastasis through platelets (Table 1). Thrombin enhances tumor cell-induced platelet aggregation (TCIPA) in vitro by fully activating specific membrane receptors on platelets (83). Treating mice with established melanoma using r-hirudin, a highly specific antagonist of thrombin, blocks coagulation events and inhibits lung metastasis (84).
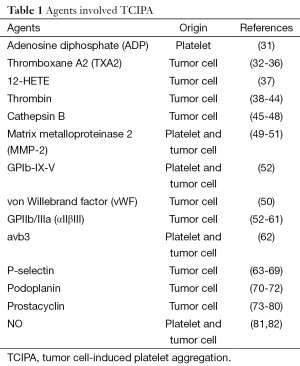
Full table
Recently, the role of platelets in the progression of malignant tumors has gained attention (85,86). Activated platelets are a primary source of lysophosphatidic acid (LPA), a simple lipid with growth factor-like signaling properties (87,88). Levels of LPA increase in up to 90% of patients with gynecologic cancers (89). LPA is involved in the initiation and progression of several cancers, such as colon, ovarian, prostate, breast, melanoma and thyroid (90,91). The effects of LPA are mediated by at least six different G protein-coupled receptors (LPA1-6) (92). Selective blockage of LPA1 and LPA2 inhibit cancer cell proliferation and invasion, which are essential for CTC generation (93-95). LPA up-regulates the activity of MMP2, MMP7 and MMP9 in cancer cells (96-99). MMPs are a family of zinc-dependent endopeptidases that are important mediators of cancer progression. They act via the degradation and remodulation of the ECM (100). The increased expression of MMPs helps tumor cells detach from the primary site and enter into the circulatory system (101). In addition, a tumor bearing mouse model with thrombocytopenia exhibits reduced tumor cell proliferation and increased tumor necrosis (102). Accumulating evidence indicates that the selective inhibition of platelet activity in patients with malignant tumors not only reduces the risk of embolic events but also reduces tumor growth (Figure 1) (103-106).
Although considerable progress has occurred in elucidating the interactions between platelets and tumor cells, there is no direct evidence that platelets affect tumor cell intravasation directly (12). Further experimentation is required to identify the mechanisms underlying this key step during metastasis.
Platelets and CTC survival
After leaving the supportive microenvironment, CTCs face many survival challenges in the circulation, including immunological attack, shear forces and apoptosis. Although the majority of CTCs are destroyed, less than 0.1% of CTCs survives and triggers TCIPA by direct contact or through the release of agonistic mediators, such as ADP, thrombin, TXA2 and tumor-associated proteinases (14,107-109). Platelets are activated in TCIPA and attach to the surface of CTCs by a GPIIb-IIIa-fibrinogen bridge and up-regulated P-selectin (Figure 2) (14,110).
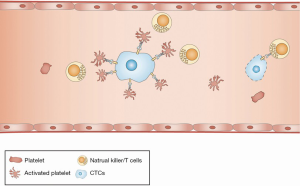
However, the molecular mechanism by which platelets promote the survival of CTCs in the blood stream is not fully understood. Several hypotheses propose that the surface coating of platelets may serve as a shield against immune assault because the effect of anti-tumor attacks mediated by NK cells is primarily based on the direct interaction with CTCs (111,112). There is solid experimental evidence that thrombocytopenia caused by either platelet depletion with anti-platelet sera or by defective platelet production significantly enhances the ability of NK cells to lyse CTCs in vitro and in vivo (113). Furthermore, activated platelets can transfer the major histocompatibility complex (MHC) to CTCs, which in turn mimics host cells and escapes immune surveillance (114). Moreover, platelet-derived TGF-β may reduce the expression of the immunoreceptor NKG2D, thus inhibiting NK cell activity (115). In addition to NK cells, platelet-derived VEGF may inhibit the maturation of dendritic cells, the major antigen-presenting cells in the immune system (116).
EMT, as well as its reverse process, MET, play pivotal roles in cancer metastasis by endowing tumorous cells with migratory, invasive and anti-apoptosis properties (117). CTCs share many phenotypic and functional traits with cells undergoing EMT (118). Recent studies suggest that CTCs in patients with breast cancer or prostate cancer co-express EMT-related markers, including E-cadherin, cytokeratin (CK), vimentin and N-cadherin (119-121). Inhibition of EMT-related signaling elements, such as Twist, Zeb and Snail, can prevent metastatic relapse (122). However, the underlying molecular mechanisms by which CTCs maintain the EMT state have not been elucidated. In addition to their well-established role in protecting CTCs from immune assaults, platelets may also contribute to the EMT of CTCs (123). TCIPA promotes platelets to release α-granules, which contain TGF-β and platelet-derived growth factor (PDGF) at concentrations several-fold higher than most cell types (124). Platelet-derived TGF-β activates the Smad signaling pathway and promotes the transdifferentiation of CTCs into a mesenchymal-like phenotype (123). PDGF is another important EMT driver that contributes to cancer invasion and angiogenesis. Overexpression of PDGF-D promotes the EMT of prostate cancer cells both in vitro and in vivo via the activation of rapamycin downstream targets, S6K and 4E-BP1 (125). The crosstalk between PDGF and EMT-related signaling pathways, such as the nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) and chemokine (C-X-C motif) receptor 4 (CXCR4), further indicates that PDGF plays an important role in EMT (126,127). Interestingly, in a study of hepatocellular carcinoma (HCC), PDGF was hypothesized to be involved in TGF-β-induced EMT of metastasizing cancer cells (128). Additional studies determined the molecular mechanism underlying this process, by which TGF-β enhances the expression of PDGF and PDGFR via activation of β-catenin and the signal transducer and activator of transcription 3 (STAT3) (129,130).
Platelet-mediated tumor extravasation
After CTCs successfully escape from physical and immune destruction in the circulation, they localize in distant organs. CTCs must anchor to the luminal side of vascular endothelial cells and then break through the subepithelial extracellular matrix (ECM) (16). Although this process is primarily mediated by the interaction between adhesion receptors on CTCs and ECs, platelets may serve as a potent regulator of this process (Figure 3) (14). First, interactions between CTCs and platelets and leukocyte activated vascular ECs induce the expression of C-C chemokine ligand 5 (CCL5), which in turn leads to the increased recruitment of leukocytes to CTCs (131). Indeed, leukocytes are implicated in promoting tumor cell survival and metastasis to the lung (132). Inhibition of CCL5 by a receptor antagonist significantly inhibits this metastatic process (131). Second, by triggering several specific signaling pathways, platelet-derived TGF-β and PDGF induce EMT in CTCs. EMT improves the ability of CTCs to avoid apoptosis and pass through the vessel wall, as described in the previous section. Ablation of platelet-derived TGF-β reduces metastasis, suggesting that they play an important role in this process (123). Third, activated platelets may be involved in the establishment of a prometastatic microenvironment through the recruitment of inflammatory cells. Upregulation of CCL2 expression in CTCs in response to the interaction with platelets promotes both monocyte recruitment and an increase in vascular permeability (133).
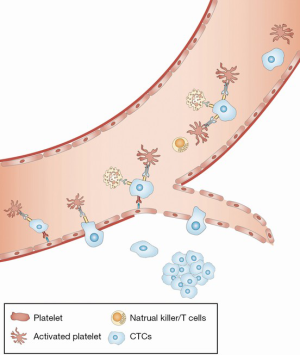
Conclusion and future directions
In conclusion, it is clear that platelets have many roles in tumor metastasis. Platelet-derived cytokines and receptors are important for protecting CTCs from host immune attack and physical stress. Because platelet-tumor cell interactions induce platelet activation and aggregation, it is reasonable to interfere with this process as a therapeutic intervention. Blockade of GPIIb/IIIa with the monoclonal antibody 10E5 reduces lung metastatic events (134). Hirudin, a specific thrombin inhibitor, inhibits metastasis in experimental models (84). Several studies suggest that heparin, a powerful P-selectin inhibitor, can attenuate tumor metastasis in mice (135). Recently, evidence has shown that the chemotherapeutic effects of aspirin on the metastatic process may depend on the inhibition of platelet function (136). Therefore, platelets are a promising therapeutic target for the attenuation of metastatic events. However, whether patients with cancer will benefit from prophylactic dose of platelet inhibitors has yet to be determined. Although prostacyclin, one of the most potent platelet inhibitors, reduces the metastasis of osteogenic sarcoma, it fails to reduce pulmonary metastasis induced by many types of tumors (137,138). Clinical studies suggest that the daily administration of semuloparin, an ultra-low-molecular-weight heparin, has no significant effect on the mortality of patients with metastatic or locally advanced solid tumors (139,140). These contradictory results suggest that the mechanism underlying platelet-involved metastasis has been only partially elucidated and is likely to be multifactorial, and several issues remain for anti-platelet therapy.
Unfortunately, although many studies have focused on this field during the last decades, significant challenges remain to be overcome before a platelet-targeted therapeutic strategy can be used in humans. Despite technical advances in the detection of CTCs, our ability to explore platelet-CTCs interactions in vivo is limited because of the shortage of materials. The majority of cancer patients have fewer than ten CTCs per milliliter of blood, and these CTCs are difficult to purify (141). Therefore, in vivo experiments are always performed in established rodent models by injecting human cancer cells into the tail vein. However, it would be ideal to study platelet-CTCs interactions from the initiation of metastasis, rather than after intravascular injection. Moreover, the CTC-associated recruitment of inflammatory cells is not established in these immunocompromised mice (16). The process of TCIPA likely involves several important platelet receptors. Experimental blockage of these receptors results in the inhibition of cancer metastasis (142). However, several studies provided contradictory results, suggesting that the number of metastatic foci increased significantly in vWF-null mice. One hypothesis proposes that the blockade of a given receptor on a platelet may be compensated for by other signaling pathways (142). Physiologically, platelets are best known for maintaining hemostasis. However, several platelet receptors, such as P-selectin, are also expressed on other normal cells. Therefore, the potential side effects of platelet-targeted compounds must be carefully evaluated. Furthermore, a better molecular understanding of platelet-CTC interactions is needed to identify individual therapeutic strategies for patients in high-risk situations for cancer metastasis.
Acknowledgements
Funding: This study was supported by grants from the National Natural Science Foundation of China (No. 81300347), the Natural Science Foundation of Jiangxi Province, China (No. 20132BAB205037, 20151BAB215008, 20151BBG70200), and Foundation of Jiangxi Educational Committee (No. GJJ14192), Foundation of Health and Family Planning Commission of Jiangxi Province (No. 20155592, 20155103).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [PubMed]
- Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest 2009;119:1420-8. [PubMed]
- Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 2004;351:781-91. [PubMed]
- Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell 2008;14:818-29. [PubMed]
- Kahn HJ, Presta A, Yang LY, et al. Enumeration of circulating tumor cells in the blood of breast cancer patients after filtration enrichment: correlation with disease stage. Breast Cancer Res Treat 2004;86:237-47. [PubMed]
- Francis JL, Biggerstaff J, Amirkhosravi A. Hemostasis and malignancy. Semin Thromb Hemost 1998;24:93-109. [PubMed]
- Sierko E, Wojtukiewicz MZ. Platelets and angiogenesis in malignancy. Semin Thromb Hemost 2004;30:95-108. [PubMed]
- Noble S, Pasi J. Epidemiology and pathophysiology of cancer-associated thrombosis. Br J Cancer 2010;102:S2-9. [PubMed]
- Blom JW, Doggen CJ, Osanto S, et al. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA 2005;293:715-22. [PubMed]
- Heit JA, Silverstein MD, Mohr DN, et al. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med 2000;160:809-15. [PubMed]
- Rendu F, Brohard-Bohn B. The platelet release reaction: granules' constituents, secretion and functions. Platelets 2001;12:261-73. [PubMed]
- Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer 2011;11:123-34. [PubMed]
- Li J, King MR. Adhesion receptors as therapeutic targets for circulating tumor cells. Front Oncol 2012;2:79. [PubMed]
- Stegner D, Dütting S, Nieswandt B. Mechanistic explanation for platelet contribution to cancer metastasis. Thromb Res 2014;133:S149-57. [PubMed]
- Nguyen DX, Bos PD, Massagué J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer 2009;9:274-84. [PubMed]
- Reymond N, d'Água BB, Ridley AJ. Crossing the endothelial barrier during metastasis. Nat Rev Cancer 2013;13:858-70. [PubMed]
- Kim MY, Oskarsson T, Acharyya S, et al. Tumor self-seeding by circulating cancer cells. Cell 2009;139:1315-26. [PubMed]
- Carmeliet P. VEGF as a key mediator of angiogenesis in cancer. Oncology 2005;69:4-10. [PubMed]
- Rafii S, Avecilla ST, Jin DK. Tumor vasculature address book: identification of stage-specific tumor vessel zip codes by phage display. Cancer Cell 2003;4:331-3. [PubMed]
- Goubran HA, Stakiw J, Radosevic M, et al. Platelets effects on tumor growth. Semin Oncol 2014;41:359-69. [PubMed]
- Brill A, Elinav H, Varon D. Differential role of platelet granular mediators in angiogenesis. Cardiovasc Res 2004;63:226-35. [PubMed]
- Verheul HM, Jorna AS, Hoekman K, et al. Vascular endothelial growth factor-stimulated endothelial cells promote adhesion and activation of platelets. Blood 2000;96:4216-21. [PubMed]
- Brock TA, Dvorak HF, Senger DR. Tumor-secreted vascular permeability factor increases cytosolic Ca2+ and von Willebrand factor release in human endothelial cells. Am J Pathol 1991;138:213-21. [PubMed]
- Ma L, Perini R, McKnight W, et al. Proteinase-activated receptors 1 and 4 counter-regulate endostatin and VEGF release from human platelets. Proc Natl Acad Sci U S A 2005;102:216-20. [PubMed]
- Italiano JE Jr, Richardson JL, Patel-Hett S, et al. Angiogenesis is regulated by a novel mechanism: pro- and antiangiogenic proteins are organized into separate platelet alpha granules and differentially released. Blood 2008;111:1227-33. [PubMed]
- Horstman LL, Ahn YS. Platelet microparticles: a wide-angle perspective. Crit Rev Oncol Hematol 1999;30:111-42. [PubMed]
- George JN, Pickett EB, Heinz R. Platelet membrane microparticles in blood bank fresh frozen plasma and cryoprecipitate. Blood 1986;68:307-9. [PubMed]
- Brill A, Dashevsky O, Rivo J, et al. Platelet-derived microparticles induce angiogenesis and stimulate post-ischemic revascularization. Cardiovasc Res 2005;67:30-8. [PubMed]
- Kim HK, Song KS, Park YS, et al. Elevated levels of circulating platelet microparticles, VEGF, IL-6 and RANTES in patients with gastric cancer: possible role of a metastasis predictor. Eur J Cancer 2003;39:184-91. [PubMed]
- Varon D, Hayon Y, Dashevsky O, et al. Involvement of platelet derived microparticles in tumor metastasis and tissue regeneration. Thromb Res 2012;130:S98-9. [PubMed]
- Alonso-Escolano D, Strongin AY, Chung AW, et al. Membrane type-1 matrix metalloproteinase stimulates tumour cell-induced platelet aggregation: role of receptor glycoproteins. Br J Pharmacol 2004;141:241-52. [PubMed]
- Grignani G, Pacchiarini L, Almasio P, et al. Characterization of the platelet-aggregating activity of cancer cells with different metastatic potential. Int J Cancer 1986;38:237-44. [PubMed]
- Grignani G, Pacchiarini L, Ricetti MM, et al. Mechanisms of platelet activation by cultured human cancer cells and cells freshly isolated from tumor tissues. Invasion Metastasis 1989;9:298-309. [PubMed]
- Pacchiarini L, Zucchella M, Milanesi G, et al. Thromboxane production by platelets during tumor cell-induced platelet activation. Invasion Metastasis 1991;11:102-9. [PubMed]
- Tzanakakis GN, Krambovitis E, Tsatsakis AM, et al. The preventive effect of ketoconazole on experimental metastasis from a human pancreatic carcinoma may be related to its effect on prostaglandin synthesis. Int J Gastrointest Cancer 2002;32:23-30. [PubMed]
- de Leval X, Benoit V, Delarge J, et al. Pharmacological evaluation of the novel thromboxane modulator BM-567 (II/II). Effects of BM-567 on osteogenic sarcoma-cell-induced platelet aggregation. Prostaglandins Leukot Essent Fatty Acids 2003;68:55-9. [PubMed]
- Steinert BW, Tang DG, Grossi IM, et al. Studies on the role of platelet eicosanoid metabolism and integrin alpha IIb beta 3 in tumor-cell-induced platelet aggregation. Int J Cancer 1993;54:92-101. [PubMed]
- Kahn ML, Nakanishi-Matsui M, Shapiro MJ, et al. Protease-activated receptors 1 and 4 mediate activation of human platelets by thrombin. J Clin Invest 1999;103:879-87. [PubMed]
- Coughlin SR. Thrombin signalling and protease-activated receptors. Nature 2000;407:258-64. [PubMed]
- Chung AW, Jurasz P, Hollenberg MD, et al. Mechanisms of action of proteinase-activated receptor agonists on human platelets. Br J Pharmacol 2002;135:1123-32. [PubMed]
- Bastida E, Escolar G, Ordinas A, et al. Morphometric evaluation of thrombogenesis by microvesicles from human tumor cell lines with thrombin-dependent (U87MG) and adenosine diphosphate-dependent (SKNMC) platelet-activating mechanisms. J Lab Clin Med 1986;108:622-7. [PubMed]
- Esumi N, Todo S, Imashuku S. Platelet aggregating activity mediated by thrombin generation in the NCG human neuroblastoma cell line. Cancer Res 1987;47:2129-35. [PubMed]
- Heinmöller E, Schropp T, Kisker O, et al. Tumor cell-induced platelet aggregation in vitro by human pancreatic cancer cell lines. Scand J Gastroenterol 1995;30:1008-16. [PubMed]
- Heinmöller E, Weinel RJ, Heidtmann HH, et al. Studies on tumor-cell-induced platelet aggregation in human lung cancer cell lines. J Cancer Res Clin Oncol 1996;122:735-44. [PubMed]
- Honn KV, Cavanaugh P, Evens C, et al. Tumor cell-platelet aggregation: induced by cathepsin B-like proteinase and inhibited by prostacyclin. Science 1982;217:540-2. [PubMed]
- Falanga A, Gordon SG. Isolation and characterization of cancer procoagulant: a cysteine proteinase from malignant tissue. Biochemistry 1985;24:5558-67. [PubMed]
- Donati MB, Gambacorti-Passerini C, Casali B, et al. Cancer procoagulant in human tumor cells: evidence from melanoma patients. Cancer Res 1986;46:6471-4. [PubMed]
- Olas B, Wachowicz B, Mielicki WP, et al. Free radicals are involved in cancer procoagulant-induced platelet activation. Thromb Res 2000;97:169-75. [PubMed]
- Jurasz P, Sawicki G, Duszyk M, et al. Matrix metalloproteinase 2 in tumor cell-induced platelet aggregation: regulation by nitric oxide. Cancer Res 2001;61:376-82. [PubMed]
- Jurasz P, Stewart MW, Radomski A, et al. Role of von Willebrand factor in tumour cell-induced platelet aggregation: differential regulation by NO and prostacyclin. Br J Pharmacol 2001;134:1104-12. [PubMed]
- Jurasz P, Chung AW, Radomski A, et al. Nonremodeling properties of matrix metalloproteinases: the platelet connection. Circ Res 2002;90:1041-3. [PubMed]
- Oleksowicz L, Mrowiec Z, Schwartz E, et al. Characterization of tumor-induced platelet aggregation: the role of immunorelated GPIb and GPIIb/IIIa expression by MCF-7 breast cancer cells. Thromb Res 1995;79:261-74. [PubMed]
- Chopra H, Hatfield JS, Chang YS, et al. Role of tumor cytoskeleton and membrane glycoprotein IRGpIIb/IIIa in platelet adhesion to tumor cell membrane and tumor cell-induced platelet aggregation. Cancer Res 1988;48:3787-800. [PubMed]
- Grossi IM, Hatfield JS, Fitzgerald LA, et al. Role of tumor cell glycoproteins immunologically related to glycoproteins Ib and IIb/IIIa in tumor cell-platelet and tumor cell-matrix interactions. FASEB J 1988;2:2385-95. [PubMed]
- Karpatkin S, Pearlstein E, Ambrogio C, et al. Role of adhesive proteins in platelet tumor interaction in vitro and metastasis formation in vivo. J Clin Invest 1988;81:1012-9. [PubMed]
- Boukerche H, Berthier-Vergnes O, Bailly M, et al. A monoclonal antibody (LYP18) directed against the blood platelet glycoprotein IIb/IIIa complex inhibits human melanoma growth in vivo. Blood 1989;74:909-12. [PubMed]
- Honn KV, Chen YQ, Timar J, et al. Alpha IIb beta 3 integrin expression and function in subpopulations of murine tumors. Exp Cell Res 1992;201:23-32. [PubMed]
- Clezardin P, Drouin J, Morel-Kopp MC, et al. Role of platelet membrane glycoproteins Ib/IX and IIb/IIIa, and of platelet alpha-granule proteins in platelet aggregation induced by human osteosarcoma cells. Cancer Res 1993;53:4695-700. [PubMed]
- Cohen SA, Trikha M, Mascelli MA. Potential future clinical applications for the GPIIb/IIIa antagonist, abciximab in thrombosis, vascular and oncological indications. Pathol Oncol Res 2000;6:163-74. [PubMed]
- Trikha M, Zhou Z, Timar J, et al. Multiple roles for platelet GPIIb/IIIa and alphavbeta3 integrins in tumor growth, angiogenesis, and metastasis. Cancer Res 2002;62:2824-33. [PubMed]
- Amirkhosravi A, Mousa SA, Amaya M, et al. Inhibition of tumor cell-induced platelet aggregation and lung metastasis by the oral GpIIb/IIIa antagonist XV454. Thromb Haemost 2003;90:549-54. [PubMed]
- Felding-Habermann B, O'Toole TE, Smith JW, et al. Integrin activation controls metastasis in human breast cancer. Proc Natl Acad Sci U S A 2001;98:1853-8. [PubMed]
- Stone JP, Wagner DD. P-selectin mediates adhesion of platelets to neuroblastoma and small cell lung cancer. J Clin Invest 1993;92:804-13. [PubMed]
- Pottratz ST, Hall TD, Scribner WM, et al. P-selectin-mediated attachment of small cell lung carcinoma to endothelial cells. Am J Physiol 1996;271:L918-23. [PubMed]
- Iwamura T, Caffrey TC, Kitamura N, et al. P-selectin expression in a metastatic pancreatic tumor cell line (SUIT-2). Cancer Res 1997;57:1206-12. [PubMed]
- Kim YJ, Borsig L, Varki NM, et al. P-selectin deficiency attenuates tumor growth and metastasis. Proc Natl Acad Sci U S A 1998;95:9325-30. [PubMed]
- Kim YJ, Borsig L, Han HL, et al. Distinct selectin ligands on colon carcinoma mucins can mediate pathological interactions among platelets, leukocytes, and endothelium. Am J Pathol 1999;155:461-72. [PubMed]
- Varki A, Varki NM. P-selectin, carcinoma metastasis and heparin: novel mechanistic connections with therapeutic implications. Braz J Med Biol Res 2001;34:711-7. [PubMed]
- Wahrenbrock M, Borsig L, Le D, et al. Selectin-mucin interactions as a probable molecular explanation for the association of Trousseau syndrome with mucinous adenocarcinomas. J Clin Invest 2003;112:853-62. [PubMed]
- Fujita N, Takagi S. The impact of Aggrus/podoplanin on platelet aggregation and tumour metastasis. J Biochem 2012;152:407-13. [PubMed]
- Kato Y, Kaneko M, Sata M, et al. Enhanced expression of Aggrus (T1alpha/podoplanin), a platelet-aggregation-inducing factor in lung squamous cell carcinoma. Tumour Biol 2005;26:195-200. [PubMed]
- Nakazawa Y, Takagi S, Sato S, et al. Prevention of hematogenous metastasis by neutralizing mice and its chimeric anti-Aggrus/podoplanin antibodies. Cancer Sci 2011;102:2051-7. [PubMed]
- Honn KV, Bockman RS, Marnett LJ. Prostaglandins and cancer: a review of tumor initiation through tumor metastasis. Prostaglandins 1981;21:833-64. [PubMed]
- Honn KV, Cicone B, Skoff A. Prostacyclin: a potent antimetastatic agent. Science 1981;212:1270-2. [PubMed]
- Lerner WA, Pearlstein E, Ambrogio C, et al. A new mechanism for tumor induced platelet aggregation. Comparison with mechanisms shared by other tumor with possible pharmacologic strategy toward prevention of metastases. Int J Cancer 1983;31:463-9. [PubMed]
- Menter DG, Onoda JM, Taylor JD, et al. Effects of prostacyclin on tumor cell-induced platelet aggregation. Cancer Res 1984;44:450-6. [PubMed]
- Menter DG, Onoda JM, Moilanen D, et al. Inhibition by prostacyclin of the tumor cell-induced platelet release reaction and platelet aggregation. J Natl Cancer Inst 1987;78:961-9. [PubMed]
- Longenecker GL, Beyers BJ, Bowen RJ, et al. Human rhabdosarcoma cell-induced aggregation of blood platelets. Cancer Res 1989;49:16-9. [PubMed]
- Schneider MR, Schillinger E, Schirner M, et al. Effects of prostacyclin analogues in in vivo tumor models. Adv Prostaglandin Thromboxane Leukot Res 1991;21B:901-8. [PubMed]
- Schirner M, Schneider MR. The prostacyclin analogue cicaprost inhibits metastasis of tumours of R 3327 MAT Lu prostate carcinoma and SMT 2A mammary carcinoma. J Cancer Res Clin Oncol 1992;118:497-501. [PubMed]
- Radomski MW, Jenkins DC, Holmes L, et al. Human colorectal adenocarcinoma cells: differential nitric oxide synthesis determines their ability to aggregate platelets. Cancer Res 1991;51:6073-8. [PubMed]
- Jenkins DC, Charles IG, Baylis SA, et al. Human colon cancer cell lines show a diverse pattern of nitric oxide synthase gene expression and nitric oxide generation. Br J Cancer 1994;70:847-9. [PubMed]
- Nierodzik ML, Plotkin A, Kajumo F, et al. Thrombin stimulates tumor-platelet adhesion in vitro and metastasis in vivo. J Clin Invest 1991;87:229-36. [PubMed]
- Esumi N, Fan D, Fidler IJ. Inhibition of murine melanoma experimental metastasis by recombinant desulfatohirudin, a highly specific thrombin inhibitor. Cancer Res 1991;51:4549-56. [PubMed]
- Boucharaba A, Serre CM, Grès S, et al. Platelet-derived lysophosphatidic acid supports the progression of osteolytic bone metastases in breast cancer. J Clin Invest 2004;114:1714-25. [PubMed]
- Belloc C, Lu H, Soria C, et al. The effect of platelets on invasiveness and protease production of human mammary tumor cells. Int J Cancer 1995;60:413-7. [PubMed]
- Gerrard JM, Robinson P. Identification of the molecular species of lysophosphatidic acid produced when platelets are stimulated by thrombin. Biochim Biophys Acta 1989;1001:282-5. [PubMed]
- Eichholtz T, Jalink K, Fahrenfort I, et al. The bioactive phospholipid lysophosphatidic acid is released from activated platelets. Biochem J 1993;291:677-80. [PubMed]
- Sutphen R, Xu Y, Wilbanks GD, et al. Lysophospholipids are potential biomarkers of ovarian cancer. Cancer Epidemiol Biomarkers Prev 2004;13:1185-91. [PubMed]
- Merchant TE, Kasimos JN, de Graaf PW, et al. Phospholipid profiles of human colon cancer using 31P magnetic resonance spectroscopy. Int J Colorectal Dis 1991;6:121-6. [PubMed]
- Mills GB, Moolenaar WH. The emerging role of lysophosphatidic acid in cancer. Nat Rev Cancer 2003;3:582-91. [PubMed]
- Leblanc R, Peyruchaud O. New insights into the autotaxin/LPA axis in cancer development and metastasis. Exp Cell Res 2015;333:183-9. [PubMed]
- Boucharaba A, Serre CM, Guglielmi J, et al. The type 1 lysophosphatidic acid receptor is a target for therapy in bone metastases. Proc Natl Acad Sci U S A 2006;103:9643-8. [PubMed]
- David M, Ribeiro J, Descotes F, et al. Targeting lysophosphatidic acid receptor type 1 with Debio 0719 inhibits spontaneous metastasis dissemination of breast cancer cells independently of cell proliferation and angiogenesis. Int J Oncol 2012;40:1133-41. [PubMed]
- Yu S, Murph MM, Lu Y, et al. Lysophosphatidic acid receptors determine tumorigenicity and aggressiveness of ovarian cancer cells. J Natl Cancer Inst 2008;100:1630-42. [PubMed]
- Fishman DA, Liu Y, Ellerbroek SM, et al. Lysophosphatidic acid promotes matrix metalloproteinase (MMP) activation and MMP-dependent invasion in ovarian cancer cells. Cancer Res 2001;61:3194-9. [PubMed]
- Jeong KJ, Park SY, Cho KH, et al. The Rho/ROCK pathway for lysophosphatidic acid-induced proteolytic enzyme expression and ovarian cancer cell invasion. Oncogene 2012;31:4279-89. [PubMed]
- Park SY, Jeong KJ, Panupinthu N, et al. Lysophosphatidic acid augments human hepatocellular carcinoma cell invasion through LPA1 receptor and MMP-9 expression. Oncogene 2011;30:1351-9. [PubMed]
- Hope JM, Wang FQ, Whyte JS, et al. LPA receptor 2 mediates LPA-induced endometrial cancer invasion. Gynecol Oncol 2009;112:215-23. [PubMed]
- Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 2001;17:463-516. [PubMed]
- Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev 2006;25:9-34. [PubMed]
- Ho-Tin-Noé B, Goerge T, Cifuni SM, et al. Platelet granule secretion continuously prevents intratumor hemorrhage. Cancer Res 2008;68:6851-8. [PubMed]
- Hettiarachchi RJ, Smorenburg SM, Ginsberg J, et al. Do heparins do more than just treat thrombosis? The influence of heparins on cancer spread. Thromb Haemost 1999;82:947-52. [PubMed]
- Borly L, Wille-Jørgensen P, Rasmussen MS. Systematic review of thromboprophylaxis in colorectal surgery -- an update. Colorectal Dis 2005;7:122-7. [PubMed]
- Smorenburg SM, Hettiarachchi RJ, Vink R, et al. The effects of unfractionated heparin on survival in patients with malignancy--a systematic review. Thromb Haemost 1999;82:1600-4. [PubMed]
- Akl EA, van Doormaal FF, Barba M, et al. Parenteral anticoagulation may prolong the survival of patients with limited small cell lung cancer: a Cochrane systematic review. J Exp Clin Cancer Res 2008;27:4. [PubMed]
- Bastida E, Ordinas A, Giardina SL, et al. Differentiation of platelet-aggregating effects of human tumor cell lines based on inhibition studies with apyrase, hirudin, and phospholipase. Cancer Res 1982;42:4348-52. [PubMed]
- Pinto S, Gori L, Gallo O, et al. Increased thromboxane A2 production at primary tumor site in metastasizing squamous cell carcinoma of the larynx. Prostaglandins Leukot Essent Fatty Acids 1993;49:527-30. [PubMed]
- Zucchella M, Dezza L, Pacchiarini L, et al. Human tumor cells cultured "in vitro" activate platelet function by producing ADP or thrombin. Haematologica 1989;74:541-5. [PubMed]
- Gong L, Cai Y, Zhou X, et al. Activated platelets interact with lung cancer cells through P-selectin glycoprotein ligand-1. Pathol Oncol Res 2012;18:989-96. [PubMed]
- Vivier E, Ugolini S, Blaise D, et al. Targeting natural killer cells and natural killer T cells in cancer. Nat Rev Immunol 2012;12:239-52. [PubMed]
- Storkus WJ, Dawson JR. Target structures involved in natural killing (NK): characteristics, distribution, and candidate molecules. Crit Rev Immunol 1991;10:393-416. [PubMed]
- Nieswandt B, Hafner M, Echtenacher B, et al. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res 1999;59:1295-300. [PubMed]
- Placke T, Örgel M, Schaller M, et al. Platelet-derived MHC class I confers a pseudonormal phenotype to cancer cells that subverts the antitumor reactivity of natural killer immune cells. Cancer Res 2012;72:440-8. [PubMed]
- Kopp HG, Placke T, Salih HR. Platelet-derived transforming growth factor-beta down-regulates NKG2D thereby inhibiting natural killer cell antitumor reactivity. Cancer Res 2009;69:7775-83. [PubMed]
- Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer 2012;12:265-77. [PubMed]
- Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer 2009;9:239-52. [PubMed]
- Armstrong AJ, Marengo MS, Oltean S, et al. Circulating tumor cells from patients with advanced prostate and breast cancer display both epithelial and mesenchymal markers. Mol Cancer Res 2011;9:997-1007. [PubMed]
- Bednarz N, Eltze E, Semjonow A, et al. BRCA1 loss preexisting in small subpopulations of prostate cancer is associated with advanced disease and metastatic spread to lymph nodes and peripheral blood. Clin Cancer Res 2010;16:3340-8. [PubMed]
- Joosse SA, Hannemann J, Spötter J, et al. Changes in keratin expression during metastatic progression of breast cancer: impact on the detection of circulating tumor cells. Clin Cancer Res 2012;18:993-1003. [PubMed]
- Aktas B, Tewes M, Fehm T, et al. Stem cell and epithelial-mesenchymal transition markers are frequently overexpressed in circulating tumor cells of metastatic breast cancer patients. Breast Cancer Res 2009;11:R46. [PubMed]
- De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer 2013;13:97-110. [PubMed]
- Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell 2011;20:576-90. [PubMed]
- Assoian RK, Komoriya A, Meyers CA, et al. Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J Biol Chem 1983;258:7155-60. [PubMed]
- Kong D, Wang Z, Sarkar SH, et al. Platelet-derived growth factor-D overexpression contributes to epithelial-mesenchymal transition of PC3 prostate cancer cells. Stem Cells 2008;26:1425-35. [PubMed]
- Liu J, Liao S, Huang Y, et al. PDGF-D improves drug delivery and efficacy via vascular normalization, but promotes lymphatic metastasis by activating CXCR4 in breast cancer. Clin Cancer Res 2011;17:3638-48. [PubMed]
- Ahmad A, Wang Z, Kong D, et al. Platelet-derived growth factor-D contributes to aggressiveness of breast cancer cells by up-regulating Notch and NF-κB signaling pathways. Breast Cancer Res Treat 2011;126:15-25. [PubMed]
- Gotzmann J, Fischer AN, Zojer M, et al. A crucial function of PDGF in TGF-beta-mediated cancer progression of hepatocytes. Oncogene 2006;25:3170-85. [PubMed]
- Lahsnig C, Mikula M, Petz M, et al. ILEI requires oncogenic Ras for the epithelial to mesenchymal transition of hepatocytes and liver carcinoma progression. Oncogene 2009;28:638-50. [PubMed]
- Fischer AN, Fuchs E, Mikula M, et al. PDGF essentially links TGF-beta signaling to nuclear beta-catenin accumulation in hepatocellular carcinoma progression. Oncogene 2007;26:3395-405. [PubMed]
- Läubli H, Spanaus KS, Borsig L. Selectin-mediated activation of endothelial cells induces expression of CCL5 and promotes metastasis through recruitment of monocytes. Blood 2009;114:4583-91. [PubMed]
- Labelle M, Hynes RO. The initial hours of metastasis: the importance of cooperative host-tumor cell interactions during hematogenous dissemination. Cancer Discov 2012;2:1091-9. [PubMed]
- Wolf MJ, Hoos A, Bauer J, et al. Endothelial CCR2 signaling induced by colon carcinoma cells enables extravasation via the JAK2-Stat5 and p38MAPK pathway. Cancer Cell 2012;22:91-105. [PubMed]
- Nierodzik ML, Klepfish A, Karpatkin S. Role of platelets, thrombin, integrin IIb-IIIa, fibronectin and von Willebrand factor on tumor adhesion in vitro and metastasis in vivo. Thromb Haemost 1995;74:282-90. [PubMed]
- Borsig L, Wong R, Feramisco J, et al. Heparin and cancer revisited: mechanistic connections involving platelets, P-selectin, carcinoma mucins, and tumor metastasis. Proc Natl Acad Sci U S A 2001;98:3352-7. [PubMed]
- Rothwell PM, Price JF, Fowkes FG, et al. Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet 2012;379:1602-12. [PubMed]
- Mehta P, Lawson D, Ward MB, et al. Effects of thromboxane A2 inhibition on osteogenic sarcoma cell-induced platelet aggregation. Cancer Res 1986;46:5061-3. [PubMed]
- Karpatkin S, Ambrogio C, Pearlstein E. Lack of effect of in vivo prostacyclin on the development of pulmonary metastases in mice following intravenous injection of CT26 colon carcinoma, Lewis lung carcinoma, or B16 amelanotic melanoma cells. Cancer Res 1984;44:3880-3. [PubMed]
- Agnelli G, George DJ, Kakkar AK, et al. Semuloparin for thromboprophylaxis in patients receiving chemotherapy for cancer. N Engl J Med 2012;366:601-9. [PubMed]
- Akl EA, Schünemann HJ. Routine heparin for patients with cancer? One answer, more questions. N Engl J Med 2012;366:661-2. [PubMed]
- Balic M, Lin H, Williams A, et al. Progress in circulating tumor cell capture and analysis: implications for cancer management. Expert Rev Mol Diagn 2012;12:303-12. [PubMed]
- Erpenbeck L, Schön MP. Deadly allies: the fatal interplay between platelets and metastasizing cancer cells. Blood 2010;115:3427-36. [PubMed]


