Pancreatic cancer surgery: past, present, and future
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is currently the 4th leading cause of cancer deaths in the United States with 2015 projections estimating 49,000 new cases, 41,000 new deaths, and a 5-year relative survival rate of only 7% (1). For those afflicted with this terrible disease, surgery remains the only hope for cure. Unfortunately, only 15-20% of patients are candidates for surgery at the time of diagnosis and among these, median postoperative survival is <20 months with a 5-year survival of only 20% (2). However, it was not long ago that pancreatic resections were thought to be impossible and more recently still that perioperative mortality rates approached 30%. Today, pancreaticoduodenectomy (PD) is the most common procedure performed for pancreatic cancer and it is carried out routinely at high-volume centers with mortality rates <2%. It has taken over a century of persistence by pioneering surgeons, each building upon the achievements of the previous, to arrive at this point (Table 1). Thanks to their efforts, the focus has now shifted from surviving the operation to surviving the cancer and the field of pancreatic surgery is evolving to reflect that. Though the operations themselves are likely to remain largely the same, the future of pancreatic surgery lies in how, when, and in whom we perform them.
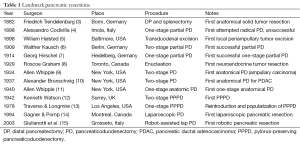
Full table
From barbers and bloodletters: the rise of surgery in the 19th century
Prior to the 19th century, the pancreas and some accounts of its disease had already been described, but abdominal surgery was uncommon and discouraged since merely entering the abdomen was almost uniformly fatal (16). Surgery was in its infancy and its practitioners, considered on par with craftsmen and artisans, held much lower social standing than their university-trained physician counterparts (17). In Europe, they aligned themselves in guilds with barbers and received training through apprenticeships. These barber-surgeons applied their broad skill with knives and razors to a range of minor external procedures (as opposed to the “internal medicine” practiced by physicians) such as lancing abscesses, excising skin lesions, and removing foreign bodies in addition to the more mundane, but steady occupations of cutting hair, shaving, and bloodletting (16).
At the dawn of the 19th century, while surgeons were still shedding their artisan roots, major surgical interventions were still relatively rare. The extraordinary pain combined with high mortality rates from postoperative infections relegated surgery to a last resort measure and emphasized speed and simplicity over technique (16). This would soon change with the revolutionary advent of anesthesia in the 1840s followed by growing adherence to Listerism in the later half of the century. These advances catalyzed the field’s transformation from a tradecraft into a true medical science capable of the complex abdominal surgery required to intervene upon the pancreas.
Ether anesthesia was first used in 1842 by a rural surgeon from Georgia named Crawford W. Long (18), but the technique was popularized by William T. G. Morton after his famous demonstration at Massachusetts General Hospital in 1846 (19,20). Absent the limitations imposed by patient discomfort, surgeons were free to dispense with slashing speed in favor of meticulousness and procedures became increasingly sophisticated. Unfortunately, these technical achievements were overshadowed by an abysmal mortality rate of over 50% for major operations (21,22). The overwhelming majority of these deaths resulted from the postoperative wound infections that developed in up to 80% of cases. At the time, the germ theory of disease was not widely accepted and surgeons did not recognize a need for cleaning instruments, hands, or even operative sites prior to surgery.
In 1867, inspired by Louis Pasteur’s experiments with fermentation, Joseph Lister published the first of his pioneering works on surgical antisepsis (23,24). He suggested that wound infection resulted from airborne contamination by ubiquitous “atmospheric germs” and recommended the use of carbolic acid in wound dressings to kill any contaminating organisms before they could cause disease. Over the next 40 years, Listerian antiseptic techniques gradually evolved into the more scientific and comprehensive principles of surgical asepsis, which sought to prevent infection by excluding bacteria altogether from the operative field (21). By the first decade of the 20th century, surgeons had assimilated most of the familiar surgical accouterments and rituals of modern aseptic technique, which led to a dramatic decline in postoperative mortality rates. German-trained New York physician Carl Beck reported in 1895 that antisepsis, followed by asepsis, had decreased amputation associated mortality at the University Hospital in Munich from an excess of 60% to just 2% (22).
The building blocks of pancreatic surgery
Cancer of the pancreas: defining the problem
In his 1761 publication The Seats and Causes of Diseases, Italian anatomist Giovanni Battista Morgagni [1682-1771] reported several cases of pancreatic “scirrhus,” which many consider to be the earliest recorded accounts of PDAC (25). However, lack of a microscopic evaluation and the ambiguous terminology of the day make it impossible to know whether his descriptions represent genuine PDAC or merely chronic pancreatitis. Additional reports begin to appear in the literature by around 1820, but perhaps the most reliable early accounts of PDAC were published in 1858 by Jacob M. Da Costa (26). His compilation of 37 cases, including the first microscopic diagnosis, helped to legitimize PDAC as a true disease entity, which even by that time had not been firmly established (27). Despite the mounting evidence confirming the existence of PDAC, efforts directed at surgical intervention were slow to develop. According to the famous Polish surgeon Johann von Mikulicz-Radecki [1850-1905], the delay in progress resulted from three seemingly insurmountable barriers that led to a noli me tangere stance toward pancreatic surgery (28). First, the anatomical location of the pancreas made it “exceedingly difficult” to access using the surgical techniques and resources available in the 19th century. Second, diagnosis of PDAC was very difficult and usually made at a late stage when disease was already unresectable. Finally, the significant morbidity of pancreatic surgery often proved fatal due to limitations in perioperative care including the lack of intravenous fluids, nutritional support, and infection control.
Surgical palliation: evolution of the bilioenteric bypass
Following the advent of surgical anesthesia and antisepsis, abdominal procedures became more frequent as surgeons were suddenly able to intervene upon previously nonsurgical diseases. It was during this period of rapid surgical discovery that many of the building blocks of modern pancreatic surgery were first developed. Notable among these is the bilioenteric bypass, which has its origins in the management of benign biliary disease before its application to malignant obstructive processes. Because the pancreas remained off limits to all but the most intrepid surgeons, palliative biliary bypass became the first form of surgical management for PDAC.
James Marion Sims [1813-1883], an American surgeon from South Carolina, performed the first planned cholecystostomy in 1878 (29). His patient was a 45-year-old woman with long-standing jaundice and a large right upper quadrant mass that he presumed to be “dropsy” of the gallbladder (gallbladder hydrops) from obstructive cholelithiasis. After noting temporary symptom relief with gallbladder aspiration, Sims decided to create a permanent fistula to allow for continuous external decompression. Under antiseptic technique, he incised the gallbladder, removed a total of 60 gallstones, and sutured the cut edges to the abdominal wall. Afterwards, the patient reportedly experienced “immediate relief of pain, itching, nausea, [and] vomiting” (29). Unfortunately, she died abruptly on post-operative day 8 from a gastrointestinal hemorrhage related to her obstructive coagulopathy. Nevertheless, Sims considered the procedure a success in principle and justified by the fact that “death is absolutely certain in every case where the gall-ducts are mechanically obstructed, unless an outlet be obtained.” Furthermore, in acknowledgement of the changing times, Sims commented that the procedure was also “a triumph for Listerism; for the post-mortem showed there was not the least trace of peritonitis or other untoward complication to be found as the direct result of the operation”.
Two years later in 1880, Alexander von Winiwarter [1848-1917] attempted the first bilioenteric bypass by performing an anastomosis between the gallbladder and colon (30). A series of anastomotic complications ensued, but eventually he was able to revise the original bypass to a functioning cholecystojejunostomy. In 1887, two surgeons independently adapted von Winiwarter’s procedure for palliation in the setting of malignancy when they performed the first planned, one-stage cholecystojejunostomies. The first was performed by the Russian surgeon Nestor Dmitrievic Monastyrski for a periampullary tumor, followed a month later by Swiss surgeon Otto Kappeler for PDAC (31).
Over time, the procedure would continue to undergo revisions and modifications, but the most significant for the evolution of pancreatic surgery came when Ambrose Monprofit performed the first Roux-en-Y cholecystojejunostomy in 1904 (32). Using an adaptation of Cesar Roux’s recently described gastrojejunostomy-en-Y technique, he fashioned a defunctionalized limb of jejunum to serve as a conduit for restoring biliodigestive continuity (33). A similar Roux-en-Y configuration with a cholecystojejunostomy biliary reconstruction would later serve as the backbone for Whipple’s revised two-stage PD (34).
The first pancreatic resections for cancer
Distal pancreatectomy (DP)
In his 1886 monograph, The Surgery of the Pancreas, preeminent American surgeon Nicolas Senn [1844-1908] wrote, “the most favorable conditions for extirpation are presented if the disease is primarily located in the tail of the pancreas” (35). Like other surgeons of the day, Senn recognized that compared to the head of the pancreas, the body and tail were more easily accessible and amenable to resection without the need for pancreatic, biliary, or gastrointestinal reconstruction. Moreover, bleeding was less of a concern because there were fewer major vascular structures in this region (apart from the splenic vessels) and tumors were less likely to cause obstructive jaundice with its attendant coagulopathy.
Based on these factors, it is no surprise that the first anatomical resection for a solid tumor of the pancreas was a DP, performed by Friedrich Trendelenburg [1844-1924] in 1882. Over the course of a 1.5-hour procedure, he resected a massive spindle cell carcinoma en bloc with the tail of the pancreas from which it arose (3). The procedure was complicated by an intraoperative splenic injury and necessitated splenectomy. Despite a postoperative course complicated by wound infection and worsening malnutrition, the patient insisted on being discharged from the hospital and reportedly died at home a few weeks later from acute respiratory failure. Unfortunately, details are scarce and no autopsy was performed to determine the specific cause of death (36,37).
Despite the patient’s poor outcome, Trendelenburg’s procedure successfully demonstrated the technical feasibility of a major pancreatic resection and marks the birth of pancreatic cancer surgery. Nevertheless, the burgeoning field remained slow to progress and over the span of more than 2 decades between 1882 to 1905, only 24 distal pancreatectomies were performed by 21 different surgeons (including Trendelenburg) (36,37).
Early attempts at pancreatic head resection
By the turn of the century, reports of pancreatic head resections for solid tumors finally began to emerge, but these were mostly limited resections like Giuseppe Ruggi’s enucleation in 1889 (38) and Domenico Biondi’s duodenum-sparing partial head resection in 1894 (39). One glaring exception is the unique case of Italian surgeon Alessandro Codivilla (1861-1912, Figure 1), who ambitiously attempted the first recorded partial PD in 1898 (4). Interestingly, Codivilla is best known for his career and contributions in the field of orthopedic surgery, but the early focus of his career, prior to appointment as professor of orthopedics, was in abdominal procedures with particular expertise in gastric surgery (4).

On exploration, Codivilla encountered an “epithelioma of the head of the pancreas” that he would have preferred to enucleate, but because it was adherent to the duodenum he decided in favor of an en bloc resection of the pancreatic head, distal stomach, proximal duodenum, and distal common bile duct. His reconstruction consisted of a Roux-en-Y gastrojejunostomy [described by Roux just 1 year prior (33)] with cholecystojejunostomy over Murphy buttons. While there is admittedly no discussion of Codivilla’s management of the pancreatic stump in the sparse documentation of the procedure, he most likely ligated it based on the typical practice of the day for distal resections and his own writings on the subject of pancreatic surgery (4). Postoperatively, the patient developed continuous drainage of serous fluid from the surgical wound followed by “milky clots” suggestive of a pancreatic fistula. The patient subsequently developed intractable diarrhea and “died of cachexia on the 21st day” (4,36).
Just 5 days after Codivilla’s procedure, William Stewart Halsted [1852-1922, Figure 2] performed the first successful resection of a periampullary cancer at the Johns Hopkins Hospital (5). Through a transduodenal approach, he resected en bloc a large wedge-shaped portion of duodenum surrounding the papillary growth with short segments of the adjacent pancreatic and common bile ducts. The ducts were then reimplanted into the duodenum by incorporating them into the primary closure of the duodenal defect. The patient survived the procedure, but ultimately died later that year from complications related to local recurrence of her cancer.
Kausch: the first successful PD
In the years following the landmark procedures by Codivilla and Halsted, a succession of discoveries paved the way for what was to be the first successful PD. The first was Theodor Kocher’s popularization of a method for duodenal mobilization in 1903 (40) followed by its successful application to pancreatic surgery by Pierre Duval in 1906 (41). The “Kocher maneuver” overcame Mikulicz’s first barrier by significantly improving surgical access to the pancreas.
In 1907, Abel Desjardins published a theoretical blueprint for a one-stage PD that included the first description of a pancreaticoenterostomy reconstruction (42,43). A year later, Louis Sauve outlined a similar procedure, but advocated for two-stages and externalization of the pancreatic remnant to form a controlled pancreatic fistula (41,42). In both cases, the authors based their reports on cadaveric dissections without ever performing them in a living person.
The American surgeon Robert Coffey built upon these contributions with his 1909 results from a series of experimental pancreaticoenterostomies performed in dogs (44). Coffey obtained his best outcomes by invaginating the pancreatic stump into a draining limb of bowel in an end-to-end fashion, surrounding the cut edge of pancreas with a protective collar of inverted, peritoneum-covered bowel.
In the same year that Coffey reported his results, German surgeon Walther Kausch [1867-1928] drew upon the cumulative knowledge gained over the preceding 11 years to perform the first successful partial PD in a patient with ampullary cancer (6). Due to severe malnutrition and obstructive jaundice, Kausch elected to perform the procedure in two stages to minimize the surgical risk. In the first, he restored biliary outflow with a loop cholecystojejunostomy and Braun anastomosis over Murphy buttons. Two months later, Kausch completed the procedure by performing an en bloc distal gastrectomy, proximal duodenectomy, and partial pancreatic head resection followed by a loop gastrojejunostomy and end-to-end pancreaticoduodenostomy in a manner similar to Coffey’s canine procedure. The patient lived an additional 9 months in good condition before ultimately dying of cholangitis.
In the 2 decades following Kausch’s procedure, there were just two additional reports of successful pancreaticoduodenal resections (7,45). Although the technical aspects of the procedure had improved greatly, diagnosis and perioperative care (two of the Mikulicz barriers to PDAC surgery) were slower to progress. Without the ability to diagnose cancer effectively at an earlier stage, surgeons were often forced to abort procedures due to advanced disease encountered upon exploration. Moreover, the inherent risks of the surgery and the limited resources available for managing even the uncomplicated cases meant that in many instances, palliative procedures had better survival than attempts at curative resection. As a result, many surgeons had abandoned efforts at resecting cancers in the head of the pancreas and periampullary cancers were resected through the largely unsuccessful transduodenal approach.
The turning point for pancreatic surgery came in 1927, just 5 years after the landmark discovery of insulin by Banting and Best (46), when Wilder and colleagues reported the first insulin-secreting tumor of the pancreas (47). Two years later, Roscoe Graham performed the first curative resection for an insulinoma by enucleation, thereby demonstrating the existence of a diagnosable pancreatic neoplasm amenable to surgical intervention (8).
From Whipple to Cameron: the modernization of pancreatic cancer surgery
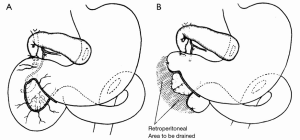
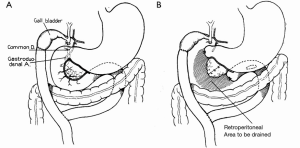
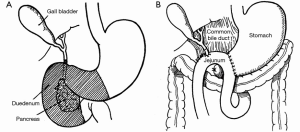
The success of pancreatic resections for neuroendocrine tumors renewed interest in pancreatic surgery, particularly in the newly appointed Surgeon-in-Chief at Columbia-Presbyterian Medical Center in New York, Allen Oldfather Whipple (1881-1963, Figure 3). At the time, he was struggling with the transduodenal approach for periampullary cancers and viewed the successes with neuroendocrine tumors as an opportunity to revive more radical resection techniques for “attacking the problem of malignancy of the pancreas and peri-ampular region.” (9). In 1935, he published his landmark manuscript entitled Treatment of Carcinoma of the Ampulla of Vater, wherein he presented a two-stage technique for the radical resection of periampullary cancers consisting of cholecystogastrostomy and posterior loop gastrojejunostomy followed by partial duodenectomy, partial pancreatic head resection, and pancreatic stump occlusion (Figure 4) (9,48). Shortly thereafter, he revised the first stage to a Roux-en-Y cholecystojejunostomy (and later choledochojejunostomy) after it became apparent that the reflux of acidic gastric contents through the cholecystogastrostomy resulted in cholangitis and anastomotic stricture (Figure 5) (34,49). After Whipple’s report on PD for ampullary tumors, Alexander Brunschig became the first to apply the procedure successfully to PDAC in 1937 (10).

In 1940, Whipple performed the first successful one-stage PD as an unplanned, but masterfully improvised procedure on a patient believed to have gastric cancer. After transecting the midportion of the stomach, Whipple was “astonished and chagrined” to find that the tumor was actually located in the head of the pancreas (11). However, because the patient was not jaundiced, he felt comfortable proceeding with an impromptu conversion to a one-stage PD. To accomplish this, he expanded the usual en bloc resection to include the distal stomach, the entire duodenum, and the pancreatic head followed by loop gastrojejunostomy and choledochojejunostomy (Figure 6) (50,51). The patient recovered uneventfully and although pathology revealed a non-functioning islet cell carcinoma, she lived an additional 9 years before succumbing to metastatic disease. Later that same year, Verne Hunt (52) in Los Angeles and Ridgway Trimble (53) in Baltimore independently performed successful one-stage pancreaticoduodenectomies as well.
Whipple had previously stressed the importance of a staged procedure to minimize the bleeding risk from prolonged biliary obstruction. Serendipitously, 1940 was also the year that vitamin K became widely available for clinical use. When combined with bile salts, it effectively reversed the coagulopathy caused by prolonged biliary obstruction. This, along with the increased availability of intraoperative blood transfusions, obviated the need for staging the operation and the one-stage procedure became the operation of choice in most patients (54).
Another of Whipple’s tenets from his early experience with PD was the avoidance of a pancreatic anastomosis in favor of stump occlusion to avoid serious anastomosis-related complications. However, by the early 1940s, several surgeons were successfully employing pancreaticoenterostomies and animal studies were demonstrating rapid epithelialization of pancreatic anastomoses within 24-48 hours. By 1942, Whipple had also incorporated an end-to-side pancreaticojejunostomy using a duct-to-mucosa technique (54). Going forward, Whipple described his procedure thus:
“(I) At least two days of vitamin K and bile salts therapy; (II) the distal half of the stomach, the entire duodenum, the terminal portion of the common duct and the head of the pancreas were removed en masse; (III) a vertical limb of the jejunum, starting at the duodenojejunal junction, was brought up through a rent in the mesocolon, behind the colon, with the following anastomoses in sequence: (i) a choledochojejunostomy, end-to-end; (ii) an anastomosis between the pancreatic duct and the wall of the jejunal opening the size of the pancreatic duct, followed by the tacking of the stump of the resected pancreas to the wall of the jejunum; (iii) an end-to-side gastrojejunostomy. A sump drain in the bed of the duodenum was used. Silk technic was employed throughout.” (11).
The “Whipple procedure” remained the standard resection technique for cancers involving the head of the pancreas until Traverso and Longmire reintroduced the concept of pylorus preservation in 1978 to reduce the incidence of postgastrectomy syndrome and marginal ulceration (13). Pylorus-preserving pancreaticoduodenectomy (PPPD) was originally described by Kenneth Watson in 1944 and consisted of a resection similar to Whipple’s original two-stage procedure with reconstruction via end-to-end duodenojejunostomy rather than loop gastrojejunostomy (12). Traverso and Longmire’s PPPD, which employed an end-to-side duodenojejunostomy, later gained popularity because of its simplified procedure, reduced operative times, and the perception that it reduced gastrectomy-related complications by preserving the stomach and pyloric sphincter mechanism. Alternatively, many believed that the more limited resection and lymphadenectomy risked leaving behind microscopic disease and that an intact sphincter increased the incidence of delayed gastric emptying (55,56). Over the years, there has been a great deal of controversy over which is the superior technique and studies comparing the two have been inconsistent and contradictory. According to a recent systematic review and meta-analysis of randomized, controlled trials comparing PPPD to classical PD, PPPD is associated with decreased blood loss and operative time, but the two procedures are otherwise equivalent in terms of mortality, morbidity, and survival (57).
Improving surgical outcomes
At the end of his career, Whipple had performed a total of 37 pancreaticoduodenectomies with a total mortality rate of approximately 33% (31). However, in contrast to the monumental progress of the 1930s and 1940s, the next 30 years were marked by failure to improve upon Whipple’s original results with reported mortality rates ranging from 20-40%, morbidity between 40-60%, and 5-year survival rates of less than 5% for PDAC (58,59). Complications ranged from post-operative hemorrhage, sepsis, intra-abdominal abscesses, delayed gastric emptying, and fistulae, all of which were usually attributed to the “Achilles’ heel” of the procedure, leakage at the pancreatic anastomosis.
During the 1960s and 1970s, the excessive mortality and lack of long-term survival led some surgeons to question whether PD should be abandoned altogether in the treatment of PDAC. In some instances, it was argued, palliative bypass alone resulted in better quality of life and longer survival (60,61). Concurrently, new pathological data were emerging to suggest that PDAC was often a multifocal disease, meaning that standard partial resections likely left disease behind in the pancreatic remnant (62-64). These factors led many in the field to advocate for total pancreatectomy (TP) over PD because it eliminated the need for the troublesome pancreatic anastomosis and addressed the issue of tumor multicentricity by providing a more oncologically radical resection. However, enthusiasm over the procedure was soon tempered as emerging studies showed that the theoretical benefits of TP had not borne out in practice. Specifically, it did not confer any survival benefit compared to partial resection, but guaranteed the additional morbidity of brittle diabetes and complete exocrine pancreatic insufficiency (65,66). Shortly thereafter, TP was generally abandoned for all but a few rare indications, such as large tumors traversing surgical boundaries.
Outcomes following PD for PDAC finally began to improve in the 1980s when several institutions reported mortality rates of <5% (67-70). This was attributed to the growing trend in centralization of care at high volume centers where surgeons specialized in pancreatic surgery. Johns Hopkins, under the leadership of John L. Cameron (Figure 7), was a leading force behind this progress and serves as the first example of the benefit of regionalization of pancreatic surgery to a high-volume institution. Between 1984 and 1995, Johns Hopkins Hospital increased its share of Maryland PDs from 21% to 59% of the total statewide volume. This was accompanied by a decline in unadjusted mortality from 3.2% [1984-1987] to 1% [1992-1995] at Johns Hopkins compared to a decline from 19.5% to 12.4% at low volume Maryland centers over the same timeframe (71). Linear regression modeling demonstrated that for every 1% increase in the hospital’s market share of PDs, the relative risk of in-hospital mortality decreased by 5% with 61% of the total observed reduction in statewide mortality attributable to regionalization. Furthermore, although mortality decreased at low volume centers as well, the relative risk increased from 4.4% to 12.6%.

The centralization of pancreatic surgery in Maryland developed out of the concerted effort to improve outcomes in PDAC. The initial successes of the Johns Hopkins group generated increasing referrals, which in turn fueled more progress. Between 1970 and 2006, 1,423 consecutive PDs were performed for PDAC at the Johns Hopkins Hospital, 80% of which were performed by just 3 surgeons and 93% by just 11 surgeons (72). During this period, case volume increased from approximately 2 to over 120 cases per year while mortality declined from 30% to 1%. As a result of this growth, the surgeons acquired increasing technical proficiency, which translated into shorter operative times and decreased intraoperative blood losses (72,73). The mounting experience at Johns Hopkins and several other developing high-volume centers allowed for the standardization of diagnostic workups, technical operative details, and postoperative management strategies into treatment algorithms and critical pathways (71).
Current trends and future directions
The safety with which pancreatic resections are now performed has led to several changes in the practice of pancreatic cancer surgery. The first major change pertains to the expanding demographic of who we operate on. Today, surgical indications are expanding to include a broader range of patients, including those with borderline resectable (BLR) cancers and those with benign precursor lesions such as intraductal papillary mucinous neoplasms (IPMNs) (74,75). Another evolving change is the manner in which we perform surgery. As our technological capabilities continue to progress, some surgeons have adopted minimally invasive alternatives to open surgery using laparoscopic and robotic techniques. The ultimate goal of these minimally invasive approaches is to maximize candidacy for adjuvant therapy and minimize the delay in its delivery by decreasing postoperative complications. However, the future of pancreatic cancer surgery and the key to attaining a truly curative outcome lies in the timely resection of disease before it has an opportunity to metastasize. This will ultimately depend on developing new and creative ways of screening for and diagnosing disease in its earliest forms.
Locally advanced and borderline resectable (BLR) disease
In contemporary practice, high-resolution tri-phasic CT imaging with three-dimensional (3-D) reconstruction is the best initial diagnostic imaging modality for PDAC. It addition to diagnosing the presence of disease, it is also the best means of determining whether it is amenable to surgical resection by evaluating for presence of metastases and involvement of major vascular structures, including the celiac axis, superior mesenteric artery (SMA), hepatic artery, superior mesenteric vein (SMV), and portal vein (PV) (76). As imaging technology has improved, it has significantly reduced the need for staging laparoscopies and the incidence of nontherapeutic laparotomies (77).
Only 15-20% of patients newly diagnosed with PDAC present with resectable disease. The majority of these patients are found to have metastases (stage IV), while another 30% have stage III disease as defined by some degree of major vessel involvement. Stage III PDAC is further divided into locally advanced unresectable pancreatic cancer (LAPC) and BLR pancreatic cancer (78). Surgically unresectable cancers are those that demonstrate metastatic spread, mesenteric or celiac arterial encasement (>180 vessel involvement), and non-reconstructable involvement of the SMV/PV (often marked by complete occlusion and extensive collateralization of flow) (79). While there is currently no single, standardized definition of BLR disease, it generally depends on whether the involved vascular structures are amenable to achieving an R0 (microscopically margin negative) resection. From a technical standpoint, resection and reconstruction of the SMV/PV can be performed safely in selected patients when performed by experienced surgeons at high-volume centers (80,81). Following en bloc vascular resection, there is no difference in disease-specific survival when compared to standard resection.
A neoadjuvant approach is most commonly applied to patients with BLR PDAC in an attempt to improve the chance of a margin-negative resection and control micrometastatic disease. In one recent study evaluating induction FOLFIRINOX [5-fluorouracil (5-FU), oxaliplatin, irinotecan, and leucovorin] therapy in LAPC, 85% of 47 patients underwent successful resection upon surgical exploration and 92% of these resulted in an R0 resection (82). Similar results have been demonstrated in small studies evaluating different neoadjuvant regimens as well (83,84).
Prophylactic surgery for benign precursors
IPMNs are relatively common macroscopic lesions of the pancreas known to be benign precursors to invasive PDAC. Like the microscopic pancreatic intraepithelial neoplasia (PanIN) lesions, IPMNs are believed to progress to PDAC through a series of genetic and morphological changes accumulated over time. Since they can be identified on imaging, they offer a unique opportunity for early detection and prevention of PDAC through surgical resection. The importance of prophylactic resection is highlighted by 5-year survival rates after resection ranging from 77-100% in patients with noninvasive lesions compared to 34-62% in patients found to have an associated invasive carcinoma (85). Guidelines currently recommend surgical resection for all main-duct IPMNs and any branch-ducts IPMNs meeting resection criteria based on specific high-risk features (85).
A trend toward minimally invasive surgery (MIS)
One of the most notable changes occurring in contemporary pancreatic cancer surgery is the trend toward MIS. MIS is currently the standard approach for many procedures such as cholecystectomy and appendectomy because it has been shown to decrease length of stay and surgical site infection rates while improving pain control and wound cosmesis (86,87). These outcomes have been replicated in more complicated abdominal and thoracic procedures as well, demonstrating that a high degree of manual dexterity can be achieved using laparoscopy. Despite early resistance stemming from concerns over safety, increased cost, and inferior oncological outcomes compared with open pancreatectomy, minimally invasive pancreatic resections are now becoming more commonplace due to the favorable results of several large studies.
Laparoscopic pancreatectomy
The first laparoscopic anatomical resection was a PD performed in 1994 by Gagner and Pomp for chronic pancreatitis (14). However, since that time there has been a much broader experience with laparoscopic DP owing to its lack of anastomoses and lesser risk of hemorrhage. To date, several studies have evaluated laparoscopic DP with splenectomy and found it to be safe and effective with morbidity and mortality rates similar to the open procedure (88-92). Moreover, there has been no decrease in long-term survival or differences in margin status, suggesting that the minimally invasive approach achieves at least an equivalent oncologic resection as the open approach (88).
The benefits of laparoscopic DP over open surgery are the same as for other procedures, including significant decreases in operative times, transfusion requirements, narcotic administration, and length of stay (88-90). Also, a metastatic evaluation of the entire abdomen can be performed at the beginning of the procedure, which can then be aborted if needed without risking any significant morbidity or mortality.
Laparoscopic PD has been more slow to develop owing to its high degree of technical difficulty, significant learning curve, and increased operative times (93,94). However, several studies have shown that when performed by experienced surgeons at specialized high-volume centers, laparoscopic PD is safe with similar morbidity or mortality as the open procedure. Specifically, there have been no reports of increased post-operative hemorrhage, delayed gastric emptying, or pancreatic fistulae as many initially feared would be the case (95-97). Furthermore, as with distal resections, oncologic outcomes are similar with no significant differences in margin status or overall survival (95-97). One study even demonstrated a statistically significant improvement in progression-free survival, though this did not carry over into overall survival (98). The benefits of laparoscopic PD are similar to those seen with laparoscopic DP and include decreased wound infection rates, transfusion requirements, and length of total hospital and intensive care unit (ICU) stay, which offset the increased cost of laparoscopic surgery (95,96,99,100).
Robotic-assisted pancreatectomy
In recent years, robotic-assisted surgery has become an increasingly popular technique in many surgical subspecialties, but only recently has been applied to pancreatic surgery. It has several technical advantages over laparoscopy including high definition 3-D visualization with up to 10× magnification, instrumentation with 7 degrees of freedom (compared to 5 for laparoscopy), tremor filtering and motion scaling for improved precision, and an ergonomic console design that minimizes muscle fatigue (101). Together, these features allow the surgeon to more closely recapitulate the technique of an open procedure, making for an easier transition to MIS compared to laparoscopy.
Though still in its infancy, robotic-assisted pancreatectomy in the form of PD, central pancreatectomy, DP, and TP have all already been described in the literature for the treatment of pancreatic adenocarcinoma (15,102). Although most series are limited to a small number of patients at select high-volume centers, they show no difference in morbidity or mortality when compared to the open approach (102-104). The largest series of 250 consecutive robotic pancreatectomies, the majority of which were for pancreatic adenocarcinoma, reported a 0.8% and 2.0% 30- and 90-day mortality, respectively (102). These rates are comparable to open and laparoscopic approaches at high-volume institutions. Additionally, conversion to an open procedure was required in only 6% of patients and overall post-operative morbidity was low. A smaller series of 134 patients undergoing robotic-assisted pancreatectomy showed similar low rates of post-operative morbidity and mortality (15). There is also literature to suggest that the robotic approach achieves better oncological resections with higher rates of negative resection margins and better lymph node yield compared to laparoscopic techniques (105).
So far, the limited experience with minimally invasive pancreatic resections has demonstrated a great deal of promise in delivering at least equivalent oncologic resections with the added benefits of speedier recovery and fewer wound-related complications. The importance of this in the larger scheme of management is the potential to increase the number of patients who qualify for adjuvant therapy and to decrease the time interval between surgery and receiving that therapy (101).
Early detection: the future of pancreatic cancer surgery
Despite all of the resources available to modern medicine today, contemporary surgeons continue to struggle with one of the same barriers Mikulicz described over a century ago; namely, the inability to diagnose PDAC early enough to make a difference (28). Pancreatic tumors are located deep within the retroperitoneum and may grow quite large before causing symptoms, at which point 80-85% of patients already have advanced unresectable disease (76). However, recent studies using mathematical models of clonal evolution within the primary tumor indicate that it may take up to 7 years for a cancer to acquire metastatic potential (106-108). If true, this offers a generous window within which an earlier diagnosis and curative resection may be obtained. In order to exploit this latency period, strategies must be developed to reliably identify and stratify at-risk populations likely to be harboring these early stage cancers. Studies have already successfully demonstrated this principle for some high-risk groups in whom magnetic resonance imaging (MRI) and endoscopic ultrasonography (EUS) were used to detect asymptomatic pancreatic lesions in up to 42% of participants (109). In 2013, the International Cancer of the Pancreas Screening (CAPS) Consortium published their screening recommendations, which focused primarily on family history and specific genetic alterations as criteria for identifying high-risk screening populations (110). However, this only covers a fraction of PDAC cases, meaning additional work is required to develop a more comprehensive strategy for identifying a broader range of high-risk patients.
New methods for screening and diagnosis will also have to be developed since many of these early cancers are likely to be too small for detection on imaging. In recent years, a great deal of research has been invested in the discovery of reliable diagnostic biomarkers for PDAC. By 2009, one study determined that over 2,500 gene products had already been suggested for this role (111). The most extensively studied of these is the sialylated blood group antigen CA19-9, which has proven utility in evaluating prognosis and recurrence, but is a poor diagnostic screening tool (112). Likewise, none of the other candidates have been applied to meaningful clinical roles in the diagnosis of PDAC either. Still, with the improving sensitivity, increasing availability, and declining cost of high-throughput sequencing technologies, there is hope on the horizon (113). A recent study by our group used next-generation sequencing to rapidly and reliably detect driver mutations from fine needle aspirates (FNA) of pancreatic cancers, while other studies have successfully detected mutant alleles such as KRAS and p53 in their serum (114-116). These studies were conducted in known, usually advanced cases of PDAC, but they effectively illustrate proof of principle. Even if early cancers and precursor lesions do not spill enough DNA into the bloodstream for detection, studies have also characterized benign pancreatic lesions by sequencing pancreatic juice and cyst fluid (117-119). Together, these results raise the possibility of using targeted deep sequencing as a viable screening method in high-risk patients. Furthermore, there is also promising research investigating new class of potential biomarkers such as circulating tumor cells, monoclonal antibodies, and miRNAs (120-122).
Summary
Pancreatic cancer is a highly lethal disease for which surgical resection offers the only hope for cure. Pancreatic resection for PDAC requires complex operations that have become safe and routine only within the past 3 decades. Our arrival at this point was made possible by the innovation and persistence of intrepid surgeons together with critical advances in related fields, such as the development of anesthesia, the germ theory of disease, and the discovery of vitamin K. Following the period of technical refinements initiated by Whipple, the contemporary era in pancreatic surgery was ushered in by the migration of care to high-volume centers of excellence. These institutions obtained improved outcomes by concentrating resources and experience, optimizing diagnostic and treatment algorithms, and effectively coordinating multidisciplinary care. Today, the field continues to evolve with the advent of minimally invasive resection techniques and the ongoing expansion of surgical indications. However, just as Mikulicz described over a century ago, the potential for a surgical cure is too often thwarted by our inability to reliably diagnose PDAC at its earliest stages. What remains for the next generation of surgeons and scientists is the development of effective methods for screening and early detection, which will dramatically increase the rate of truly curative resections.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [PubMed]
- Dal Molin M, Zhang M, de Wilde RF, et al. Very Long-term Survival Following Resection for Pancreatic Cancer Is Not Explained by Commonly Mutated Genes: Results of Whole-Exome Sequencing Analysis. Clin Cancer Res 2015;21:1944-50. [PubMed]
- Witzel O. Aus der Klinik des Herrn Prof. Trendelenburg. Beiträge zur Chirurgie der Bauchorgane. Deutsche Zeitschrift für Chirurgie 1886;24:326-54.
- Schnelldorfer T, Sarr MG. Alessandro Codivilla and the first pancreatoduodenectomy. Arch Surg 2009;144:1179-84. [PubMed]
- Halsted WS. Contributions to the surgery of the bile passages, especially of the common bile-duct. Bost Med Surg J 1899;141:645-54.
- Kausch W. Das Carcinom der Papilla duodeni und seine radikale Entfernung. Beitr Klin Chir 1912;78:439-86.
- Hirschel G. Die Resektion des Duodenums mit der Papille wegen Karzinoms. Munchen Med Wochenschr 1914;61:1728-9.
- Howland G, Campbell WR, Maltby EJ, et al. Dysinsulinism convulsions and coma due to islet cell tumor of the pancreas, with operation and cure. JAMA 1929;93:674-9.
- Whipple AO, Parsons WB, Mullins CR. Treatment of carcinoma of the ampulla of vater. Ann Surg 1935;102:763-79. [PubMed]
- Brunschwig A. Resection of head of pancreas and duodenum for carcinoma--pancreatoduodenectomy. CA Cancer J Clin 1974;24:363-7. [PubMed]
- Whipple AO. A reminiscence: pancreaticduodenectomy. Rev Surg 1963;20:221-5. [PubMed]
- Watson K. Carcinoma of ampulla of vater successful radical resection. Br J Surg 1944;31:368-73.
- Traverso LW, Longmire WP Jr. Preservation of the pylorus in pancreaticoduodenectomy. Surg Gynecol Obstet 1978;146:959-62. [PubMed]
- Gagner M, Pomp A. Laparoscopic pylorus-preserving pancreatoduodenectomy. Surg Endosc 1994;8:408-10. [PubMed]
- Giulianotti PC, Sbrana F, Bianco FM, et al. Robot-assisted laparoscopic pancreatic surgery: single-surgeon experience. Surg Endosc 2010;24:1646-57. [PubMed]
- Gawande A. Two hundred years of surgery. N Engl J Med 2012;366:1716-23. [PubMed]
- DeBakey ME. A surgical perspective. Ann Surg 1991;213:499-531. [PubMed]
- Long CW. An account of the first use of sulphuric ether by inhalation as an anaesthetic in surgical operations. South Med Surg J 1849;5:705-13.
- Bigelow HJ. Insensibility during Surgical Operations Produced by Inhalation. Boston Med Surg J 1846;35:309-17.
- Jackson CT, Morton WM, Eddy RH, et al. The Patent Letheon—Jackson and Morton's Specification. Boston Med Surg J 1847;36:194-8.
- Alexander JW. The contributions of infection control to a century of surgical progress. Ann Surg 1985;201:423-8. [PubMed]
- Beck C. A Manual of the modern theory and technique of surgical asepsis. Philadelphia: W. B. Saunders, 1895.
- Lister J. On a new method of treating compound fracture, abscess, etc. Lancet 1867;90:95-6.
- Jessney B. Joseph Lister (1827-1912): a pioneer of antiseptic surgery remembered a century after his death. J Med Biogr 2012;20:107-10. [PubMed]
- Morgagni GB. De sedibus, et causis morborum per anatomen indagatis libri quinque. Venice: Remondini, 1761.
- Da Costa J. On the morbid anatomy and symptoms of cancer of the pancreas [Extracted from the Proceedings of the Pathological Society of Philadelphia]. Philadelphia: J. B. Lippincott & Co., 1858.
- Brunschwig A. Surgery of Pancreatic Tumors. St. Louis: C. V. Mosby Co, 1942.
- Von Mikulicz-Radecki. I. Surgery of the Pancreas With Especial Consideration of Trauma and Inflammatory Processes. Ann Surg 1903;38:1-29. [PubMed]
- Sims JM. Remarks on Cholecystotomy in Dropsy of the Gall-Bladder. Br Med J 1878;1:811-5. [PubMed]
- von Winiwarter A. Ein Fall von Gallenretention betingt durch Impermeabilitat des Ductus choledochus, Anlegung einer Gallenblasen-Darmfistel, Heilung. Prager Med Wochenschr 1882;7:201.
- Howard JM. History of pancreatic head resection—the evaluation of surgical technique. Am J Surg 2007;194:S6-S10.
- Monprofit A. On Cholecystenterostomy in the form of a “Y.”. Br Med J 1908;2:991.
- Hutchison RL, Hutchison AL. César Roux and his original 1893 paper. Obes Surg 2010;20:953-6. [PubMed]
- Whipple AO. Surgical treatment of carcinoma of the ampullary region and head of the pancreas. Am J Surg 1938;40:260-3.
- Senn N. Surgery of the pancreas as based upon experiment and clinical researches. Trans Am Surg Assoc 1886;4:99-123.
- Schnelldorfer T, Adams DB, Warshaw AL, et al. Forgotten pioneers of pancreatic surgery: beyond the favorite few. Ann Surg 2008;247:191-202. [PubMed]
- Fernández-del Castillo C, Warhaw AL. Surgical Pioneers of the Pancreas. Am J Surg 2007;194:S2-S5.
- Ruggi G. Intorno ad un caso di carcinoma primitivo del pancreas, curato e guarito coll’asportazione del tumore. Napoli: Giorn Internaz Sci Med, 1890.
- Biondi D. Contributo clinico e sperimentale alla chirurgia del pancreas. Clin Chir 1896;4:131-41; 145-61.
- Kocher T. Mobilisierung des Duodenum und gastroduodenostomie. Zentralbl Chir 1903;30:33.
- Sauvé L. Des pancréatectomies et spécialement de la pancréatectomie céphalique Rev Chir 1908;37:113-52, 335-85. [On pancreatectomies and in particular on pancreatectomy of the head].
- Johnson AB. Operative Therapeusis: Volume 4. New York: Appleton, 1915.
- Technique de la Pancréatectomie Desjardins A. Rev chir 1907;35:945-73.
- Coffey RC. XVII. Pancreato-enterostomy and Pancreatectomy: A Preliminary Report. Ann Surg 1909;50:1238-64. [PubMed]
- Tenani O. Contributo alla chirurgia della papilla del Vater. Policlinico 1922;29:291-300.
- Banting FG, Best CH. The internal secretion of the pancreas. J Lab Clin Med 1922;7:251-66. [PubMed]
- Wilder RM, Allan FN, Power MH, et al. Carcinoma of the islands of the pancreas: hyperinsulinism and hypoglycemia. JAMA 1927;89:348-55.
- Whipple AO. The rationale of radical surgery for cancer of the pancreas and ampullary region. Ann Surg 1941;114:612-5. [PubMed]
- Parsons WB. Carcinoma of the pancreas and carcinoma of the ampulla of Vater: a re-evaluation; the L. Duncan Bulkley lecture. Bull N Y Acad Med 1951;27:339-50. [PubMed]
- Whipple AO. Pancreaticoduodenectomy for Islet Carcinoma: A Five-Year Follow-Up. Ann Surg 1945;121:847-52. [PubMed]
- Whipple AO. Present day surgery of the pancreas. N Engl J Med 1942;226:515-26.
- Hunt VC. Surgical management of carcinoma of the ampulla of vater and of the periampullary portion of the duodenum. Ann Surg 1941;114:570-602. [PubMed]
- Trimble IR, Parsons WB, Sherman CP. A one-stage operation for the cure of carcinoma of the ampulla of Vater and the head of the pancreas. Surg Gynecol Obstet 1941;73:711-22.
- Whipple AO. Observations on radical surgery for lesions of the pancreas. Surg Gynecol Obstet 1946;82:623-31. [PubMed]
- Warshaw AL, Torchiana DL. Delayed gastric emptying after pylorus-preserving pancreaticoduodenectomy. Surg Gynecol Obstet 1985;160:1-4. [PubMed]
- Nikfarjam M. Pylorus preserving pancreaticoduodenectomy. Saudi J Gastroenterol 2010;16:65. [PubMed]
- Diener MK, Fitzmaurice C, Schwarzer G, et al. Pylorus-preserving pancreaticoduodenectomy (pp Whipple) versus pancreaticoduodenectomy (classic Whipple) for surgical treatment of periampullary and pancreatic carcinoma. Cochrane Database Syst Rev 2011.CD006053. [PubMed]
- Lillemoe KD, Rikkers LF. Pancreaticoduodenectomy: the golden era. Ann Surg 2006;244:16-7. [PubMed]
- Lillemoe KD. Current management of pancreatic carcinoma. Ann Surg 1995;221:133-48. [PubMed]
- Crile G Jr. The advantages of bypass operations over radical pancreatoduodenectomy in the treatment of pancreatic carcinoma. Surg Gynecol Obstet 1970;130:1049-53. [PubMed]
- Shapiro TM. Adenocarcinoma of the pancreas: a statistical analysis of biliary bypass vs Whipple resection in good risk patients. Ann Surg 1975;182:715-21. [PubMed]
- Collins JJ Jr, Craighead JE, Brooks JR. Rationale for total pancreatectomy for carcinoma of the pancreatic head. N Engl J Med 1966;274:599-602. [PubMed]
- Ihse I, Lilja P, Arnesjö B, et al. Total pancreatectomy for cancer. An appraisal of 65 cases. Ann Surg 1977;186:675-80. [PubMed]
- Levin B. Panel: cancer of the pancreas. Am J Surg 1978;135:185-91. [PubMed]
- Müller MW, Friess H, Kleeff J, et al. Is there still a role for total pancreatectomy? Ann Surg 2007;246:966-74; discussion 974-5. [PubMed]
- Karpoff HM, Klimstra DS, Brennan MF, et al. Results of total pancreatectomy for adenocarcinoma of the pancreas. Arch Surg 2001;136:44-7; discussion 48. [PubMed]
- Crist DW, Sitzmann JV, Cameron JL. Improved hospital morbidity, mortality, and survival after the Whipple procedure. Ann Surg 1987;206:358-65. [PubMed]
- Grace PA, Pitt HA, Tompkins RK, et al. Decreased morbidity and mortality after pancreatoduodenectomy. Am J Surg 1986;151:141-9. [PubMed]
- Braasch JW, Deziel DJ, Rossi RL, et al. Pyloric and gastric preserving pancreatic resection. Experience with 87 patients. Ann Surg 1986;204:411-8. [PubMed]
- Kalser MH, Ellenberg SS. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg 1985;120:899-903. [PubMed]
- Gordon TA, Bowman HM, Tielsch JM, et al. Statewide regionalization of pancreaticoduodenectomy and its effect on in-hospital mortality. Ann Surg 1998;228:71-8. [PubMed]
- Winter JM, Cameron JL, Campbell KA, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: A single-institution experience. J Gastrointest Surg 2006;10:1199-210; discussion 1210-1. [PubMed]
- Yeo CJ, Cameron JL, Lillemoe KD, et al. Pancreaticoduodenectomy for cancer of the head of the pancreas. 201 patients. Ann Surg 1995;221:721-31; discussion 731-3. [PubMed]
- Gillen S, Schuster T, Meyer Zum Büschenfelde C, et al. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med 2010;7:e1000267. [PubMed]
- He J, Cameron JL, Ahuja N, et al. Is it necessary to follow patients after resection of a benign pancreatic intraductal papillary mucinous neoplasm? J Am Coll Surg 2013;216:657-65; discussion 665-7. [PubMed]
- Vincent A, Herman J, Schulick R, et al. Pancreatic cancer. Lancet 2011;378:607-20. [PubMed]
- White R, Winston C, Gonen M, et al. Current utility of staging laparoscopy for pancreatic and peripancreatic neoplasms. J Am Coll Surg 2008;206:445-50. [PubMed]
- Hidalgo M. Pancreatic cancer. N Engl J Med 2010;362:1605-17. [PubMed]
- Tempero MA, Malafa MP, Behrman SW, et al. Pancreatic adenocarcinoma, version 2.2014: featured updates to the NCCN guidelines. J Natl Compr Canc Netw 2014;12:1083-93. [PubMed]
- Tseng JF, Raut CP, Lee JE, et al. Pancreaticoduodenectomy with vascular resection: margin status and survival duration. J Gastrointest Surg 2004;8:935-49; discussion 949-50. [PubMed]
- Bockhorn M, Burdelski C, Bogoevski D, et al. Arterial en bloc resection for pancreatic carcinoma. Br J Surg 2011;98:86-92. [PubMed]
- Ferrone CR, Marchegiani G, Hong TS, et al. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann Surg 2015;261:12-7. [PubMed]
- Stokes JB, Nolan NJ, Stelow EB, et al. Preoperative capecitabine and concurrent radiation for borderline resectable pancreatic cancer. Ann Surg Oncol 2011;18:619-27. [PubMed]
- Patel M, Hoffe S, Malafa M, et al. Neoadjuvant GTX chemotherapy and IMRT-based chemoradiation for borderline resectable pancreatic cancer. J Surg Oncol 2011;104:155-61. [PubMed]
- Tanaka M, Fernández-del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology 2012;12:183-97. [PubMed]
- Sauerland S, Jaschinski T, Neugebauer EA. Laparoscopic versus open surgery for suspected appendicitis. Cochrane Database Syst Rev 2010.CD001546. [PubMed]
- Keus F, Gooszen HG, van Laarhoven CJ. Open, small-incision, or laparoscopic cholecystectomy for patients with symptomatic cholecystolithiasis. An overview of Cochrane Hepato-Biliary Group reviews. Cochrane Database Syst Rev 2010.CD008318. [PubMed]
- Venkat R, Edil BH, Schulick RD, et al. Laparoscopic distal pancreatectomy is associated with significantly less overall morbidity compared to the open technique: a systematic review and meta-analysis. Ann Surg 2012;255:1048-59. [PubMed]
- Vijan SS, Ahmed KA, Harmsen WS, et al. Laparoscopic vs open distal pancreatectomy: a single-institution comparative study. Arch Surg 2010;145:616-21. [PubMed]
- Shin SH, Kim SC, Song KB, et al. A comparative study of laparoscopic vs. open distal pancreatectomy for left-sided ductal adenocarcinoma: a propensity score-matched analysis. J Am Coll Surg 2015;220:177-85. [PubMed]
- Kooby DA, Hawkins WG, Schmidt CM, et al. A multicenter analysis of distal pancreatectomy for adenocarcinoma: is laparoscopic resection appropriate? J Am Coll Surg 2010;210:779-85, 786-7. [PubMed]
- Fernández-Cruz L, Sáenz A, Astudillo E, et al. Outcome of laparoscopic pancreatic surgery: endocrine and nonendocrine tumors. World J Surg 2002;26:1057-65. [PubMed]
- Hardacre JM. Is there a learning curve for pancreaticoduodenectomy after fellowship training? HPB Surg 2010;2010:230287.
- Speicher PJ, Nussbaum DP, White RR, et al. Defining the learning curve for team-based laparoscopic pancreaticoduodenectomy. Ann Surg Oncol 2014;21:4014-9. [PubMed]
- Asbun HJ, Stauffer JA. Laparoscopic vs open pancreaticoduodenectomy: overall outcomes and severity of complications using the Accordion Severity Grading System. J Am Coll Surg 2012;215:810-9. [PubMed]
- Lei P, Wei B, Guo W, et al. Minimally invasive surgical approach compared with open pancreaticoduodenectomy: a systematic review and meta-analysis on the feasibility and safety. Surg Laparosc Endosc Percutan Tech 2014;24:296-305. [PubMed]
- Palanivelu C, Rajan PS, Rangarajan M, et al. Evolution in techniques of laparoscopic pancreaticoduodenectomy: a decade long experience from a tertiary center. J Hepatobiliary Pancreat Surg 2009;16:731-40. [PubMed]
- Croome KP, Farnell MB, Que FG, et al. Total laparoscopic pancreaticoduodenectomy for pancreatic ductal adenocarcinoma: oncologic advantages over open approaches? Ann Surg 2014;260:633-8; discussion 638-40. [PubMed]
- Kendrick ML. Laparoscopic and robotic resection for pancreatic cancer. Cancer J 2012;18:571-6. [PubMed]
- Mesleh MG, Stauffer JA, Bowers SP, et al. Cost analysis of open and laparoscopic pancreaticoduodenectomy: a single institution comparison. Surg Endosc 2013;27:4518-23. [PubMed]
- Ongchin M, Hogg ME, Zeh HJ 3rd, et al. Essentials and Future Directions of Robotic Pancreatic Surgery. In: Kroh M, Chalikonda S, editors. Essentials of Robotic Surgery. Cham: Springer International Publishing, 2015:131-48.
- Zureikat AH, Moser AJ, Boone BA, et al. 250 robotic pancreatic resections: safety and feasibility. Ann Surg 2013;258:554-9; discussion 559-62. [PubMed]
- Buchs NC, Addeo P, Bianco FM, et al. Robotic versus open pancreaticoduodenectomy: a comparative study at a single institution. World J Surg 2011;35:2739-46. [PubMed]
- Lai EC, Yang GP, Tang CN. Robot-assisted laparoscopic pancreaticoduodenectomy versus open pancreaticoduodenectomy--a comparative study. Int J Surg 2012;10:475-9. [PubMed]
- Daouadi M, Zureikat AH, Zenati MS, et al. Robot-assisted minimally invasive distal pancreatectomy is superior to the laparoscopic technique. Ann Surg 2013;257:128-32. [PubMed]
- Yachida S, Jones S, Bozic I, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature 2010;467:1114-7. [PubMed]
- Haeno H, Gonen M, Davis MB, et al. Computational modeling of pancreatic cancer reveals kinetics of metastasis suggesting optimum treatment strategies. Cell 2012;148:362-75. [PubMed]
- Yu J, Blackford AL, Dal Molin M, et al. Time to progression of pancreatic ductal adenocarcinoma from low-to-high tumour stages. Gut 2015. [Epub ahead of print]. [PubMed]
- Canto MI, Hruban RH, Fishman EK, et al. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology 2012;142:796-804; quiz e14-5.
- Canto MI, Harinck F, Hruban RH, et al. International Cancer of the Pancreas Screening (CAPS) Consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut 2013;62:339-47. [PubMed]
- Harsha HC, Kandasamy K, Ranganathan P, et al. A compendium of potential biomarkers of pancreatic cancer. PLoS Med 2009;6:e1000046. [PubMed]
- Winter JM, Yeo CJ, Brody JR. Diagnostic, prognostic, and predictive biomarkers in pancreatic cancer. J Surg Oncol 2013;107:15-22. [PubMed]
- Lennon AM, Wolfgang CL, Canto MI, et al. The early detection of pancreatic cancer: what will it take to diagnose and treat curable pancreatic neoplasia? Cancer Res 2014;74:3381-9. [PubMed]
- Valero V 3rd, Saunders TJ, He J, et al. Reliable Detection of Somatic Mutations in Fine Needle Aspirates of Pancreatic Cancer With Next-generation Sequencing: Implications for Surgical Management. Ann Surg 2015. [Epub ahead of print]. [PubMed]
- Kahlert C, Melo SA, Protopopov A, et al. Identification of double-stranded genomic DNA spanning all chromosomes with mutated KRAS and p53 DNA in the serum exosomes of patients with pancreatic cancer. J Biol Chem 2014;289:3869-75. [PubMed]
- Bettegowda C, Sausen M, Leary RJ, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med 2014;6:224ra24.
- Singhi AD, Nikiforova MN, Fasanella KE, et al. Preoperative GNAS and KRAS testing in the diagnosis of pancreatic mucinous cysts. Clin Cancer Res 2014;20:4381-9. [PubMed]
- Shi C, Fukushima N, Abe T, et al. Sensitive and quantitative detection of KRAS2 gene mutations in pancreatic duct juice differentiates patients with pancreatic cancer from chronic pancreatitis, potential for early detection. Cancer Biol Ther 2008;7:353-360. [PubMed]
- Kanda M, Knight S, Topazian M, et al. Mutant GNAS detected in duodenal collections of secretin-stimulated pancreatic juice indicates the presence or emergence of pancreatic cysts. Gut 2013;62:1024-33. [PubMed]
- Maker AV, Carrara S, Jamieson NB, et al. Cyst fluid biomarkers for intraductal papillary mucinous neoplasms of the pancreas: a critical review from the international expert meeting on pancreatic branch-duct-intraductal papillary mucinous neoplasms. J Am Coll Surg 2015;220:243-53. [PubMed]
- Schultz NA, Dehlendorff C, Jensen BV, et al. MicroRNA biomarkers in whole blood for detection of pancreatic cancer. JAMA 2014;311:392-404. [PubMed]
- Gold DV, Newsome G, Liu D, et al. Mapping PAM4 (clivatuzumab), a monoclonal antibody in clinical trials for early detection and therapy of pancreatic ductal adenocarcinoma, to MUC5AC mucin. Mol Cancer 2013;12:143. [PubMed]




